Abstract
Meiotic silencing of unpaired chromatin (MSUC) occurs during the first meiotic prophase, as chromosomes that fail to pair are sequestered into a transcriptionally repressive nuclear domain. This phenomenon is exemplified by the heterologous sex chromosomes of male mammals, where the ATR DNA damage response kinase is crucial for this silencing event. However, the mechanisms underlying the initiation of MSUC remain unknown. Here, we show that essential components of ATR signaling in murine somatic cells are spatially confined to unpaired chromosomes in spermatocytes, including the ATR-dependent phosphorylation of the single-stranded DNA (ssDNA)-binding complex replication protein A (RPA) and the checkpoint kinase CHK1. These observations support a model in which ssDNA plays a central role in the recruitment of ATR during MSUC, and provide a link to meiotic progression through activation of CHK1.
KEY WORDS: Meiosis, Spermatogenesis, Transcriptional regulation, CHEK1, Mouse
Summary: Meiotic chromosomal regions that fail to synapse elicit meiotic silencing of unsynapsed chromatin (MSUC), which shows similarities to the DNA damage response to replication stress in somatic cells.
INTRODUCTION
Transcription of protein-coding genes from the male sex chromosomes is repressed during the first meiotic prophase. This phenomenon, which is known as meiotic sex chromosome inactivation (MSCI), is a consequence of the heterologous nature of the X and Y chromosomes. MSCI itself represents a specialized form of a mechanism that is not restricted to the sex chromosomes of males: any chromosome, or chromosomal region, that fails to pair (synapse) with a homolog will elicit an identical transcriptional response [known as meiotic silencing of unsynapsed chromatin (MSUC) (Schimenti, 2005)]. Apart from a transcriptional response, activation of this pathway is closely linked with meiotic progression and may serve as a form of quality control, ensuring that spermatocytes with excessive pairing defects are eliminated, thereby preventing the formation of aneuploid gametes (Turner, 2007). However, it remains unclear how a meiotic chromosome can be distinguished on the basis of synapsis to elicit this distinctive transcriptional and physiological response.
Upon initiation of MSCI, the sex chromosomes undergo a pronounced cytological transformation, becoming sequestered into a specialized nuclear domain (known as the XY body) and depleted of elongating RNA polymerase II. Coincidently, the XY body becomes enriched for a significant number of factors involved in somatic DNA damage responses, many of which are distinct from those utilized in the repair of meiotic double-stranded breaks (DSBs) by homologous recombination. Ablation of the genes encoding the DNA damage proteins associated with the XY body phenotypically manifest as defective or incomplete MSCI and, most often, meiotic arrest (Ichijima et al., 2012). Therefore, the DNA repair machinery appears especially significant in activating this chromosome-wide form of transcriptional repression.
Among the proteins enriched on the XY body, the ataxia telangiectasia and Rad3 related (ATR) DNA damage response kinase plays a central role in MSCI activation (Royo et al., 2013). ATR is an early marker of MSCI: as homologous chromosomes undergo synapsis during the zygotene stage, ATR becomes enriched at unpaired (asynapsed) chromosomes and is responsible for catalyzing phosphorylation of the histone variant H2AFX near the axial elements (the proteinacious structure to which meiotic chromosomes are tethered). The spreading of H2AFX phosphorylation is dependent upon ATR and mediator of DNA damage checkpoint 1 (MDC1), and is concomitant with the hallmark transcriptional silencing of MSCI (Fernandez-Capetillo et al., 2003; Ichijima et al., 2011; Royo et al., 2013). Despite its importance, the signal to which ATR is recruited during the early stages of MSCI remains undefined. Experimental evidence has suggested that initiation of MSCI is at least partially dependent on the BRCA1 protein (Turner et al., 2004; Broering et al., 2014). Together with ATR, localization of BRCA1 to asynapsed chromatin is an early event in meiosis. Deletion of exon 11 from the Brca1 gene in the male germline compromises the activation of MSCI: ATR recruitment and H2AFX phosphorylation are reduced, but not entirely ablated, leading to MSCI failure. Despite this requirement, it has been shown that other events in the early stages of MSCI, such as recruitment of the HORMAD proteins, is unaffected by the Brca1 mutation (Broering et al., 2014). Therefore, additional, BRCA1-independent mechanisms are likely to participate in ATR activation during MSCI/MSUC.
In proliferating somatic cells, ATR is robustly activated by the presence of single-stranded DNA (ssDNA) bound by the heterotrimeric complex replication protein A (RPA) (Zou and Elledge, 2003). ssDNA/RPA can be generated through a number of mechanisms and DNA lesions, including the exonucleolytic processing of DSBs in preparation for homologous recombination, and stalled replication forks, when replicative DNA polymerases become uncoupled from associated DNA helicases. Once activated, ATR phosphorylates numerous targets to promote repair of the lesion, and potentiates a response to control cell-cycle progression. Although it is unknown whether a similar mechanism elicits ATR activity during meiosis, ATR recruitment and MSUC can still occur in spermatocytes lacking the endonuclease responsible for generating meiotic DSBs, SPO11 (Bellani et al., 2005). Therefore, meiotic ATR activation appears not entirely dependent on the canonical mechanism for meiotic DSB formation. This has led to the proposal that SPO11-independent ssDNA regions underlie MSCI/MSUC initiation (Ichijima et al., 2012). In support of this hypothesis, foci of RPA and other repair proteins with affinity for ssDNA are detectable in the spermatocytes of a catalytically inactive Spo11 mutant (Carofiglio et al., 2013), implying the presence of ssDNA generated independently of SPO11-dependent DSBs in the meiotic genome. If ATR recruitment to asynapsed chromatin is dependent upon ssDNA/RPA, these foci may represent genomic sites of meiotic ATR activation during the earliest phases of MSUC/MSCI.
To understand the mechanisms of MSCI initiation, we explored the hypothesis that if meiotic ATR activation were functionally related to well-studied mechanisms of ATR recruitment in somatic cells, then the hallmarks of somatic ATR activation should be enriched on the XY body. We focused on RPA itself because it can be phosphorylated in an ATR-dependent manner during replication stress (Anantha et al., 2007; Vassin et al., 2009; Shiotani et al., 2013). Phosphorylation of serine 33 of the RPA32 (RPA2 – Mouse Genome Informatics) subunit (pRPA) by ATR serves as a sensitive reporter of replication-induced DNA damage. Here, we demonstrate that the XY and asynapsed autosomes are highly enriched for pRPA. The presence of pRPA is dependent upon ATR, but independent of SPO11, which is consistent with known parameters of MSUC/MSCI initiation. Whereas RPA/ssDNA activates ATR, the checkpoint protein CHK1 (CHEK1 – Mouse Genome Informatics) transduces ATR activation into physiological responses, including cell-cycle control and apoptosis (Liu et al., 2000). Coincident with the localization of pRPA and ATR, we demonstrate that asynapsed chromatin is similarly enriched for the active, phosphorylated forms of CHK1. Together, these data provide evidence for mechanistic similarities between MSUC and the DNA damage response to replication stress in somatic cells.
RESULTS
Phosphorylation of serine 33 of RPA32 is a marker of asynapsis
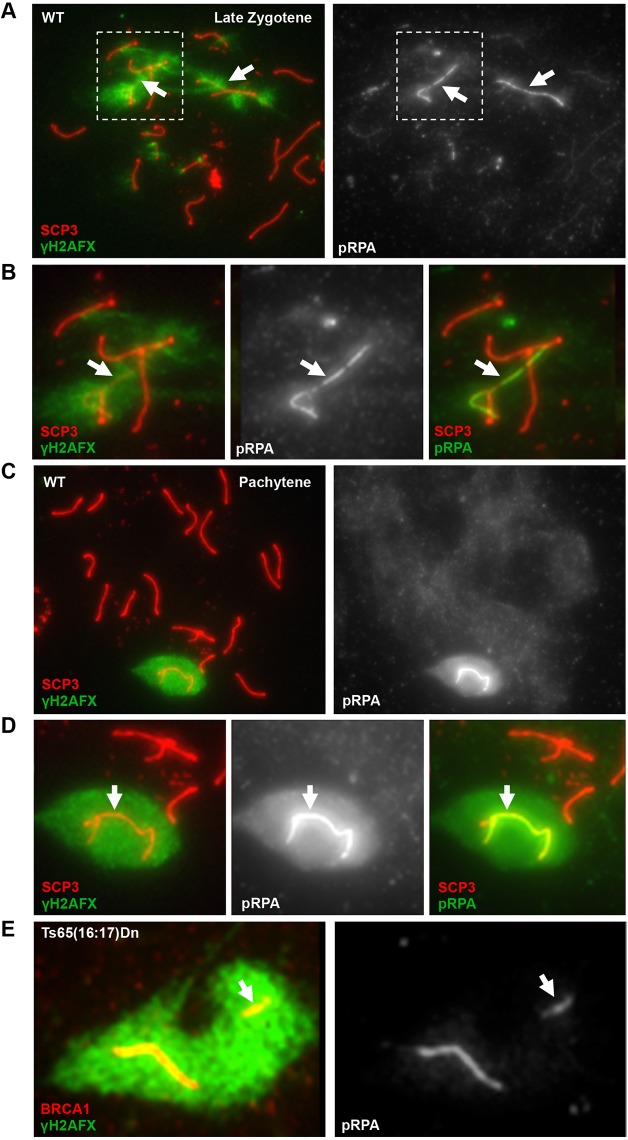
We first examined the distribution of pRPA during stages of the first meiotic prophase, relative to known markers of the XY body. MSCI/MSUC first initiates in late zygotene spermatocytes, as homologous chromosomes are completing synapsis. At this stage, ATR is recruited to asynapsed chromosomes and initiates H2AFX phosphorylation distinct from the ATM-dependent H2AFX phosphorylation of SPO11-dependent DSBs (Bellani et al., 2005). When synapsis of homologous chromosomes is completed by the pachytene stage, the X and Y chromosomes are entirely coated with phosphorylated H2AFX (γH2AFX) and retain ATR. To determine whether pRPA is involved in MSCI, we used indirect immunofluorescence to examine the dynamics of pRPA in primary spermatocytes. To identify specific stages within the first meiotic prophase, spermatocytes were co-stained with antibodies against γH2AFX and SCP3 (SYCP3 – Mouse Genome Informatics), a component of the axial element. pRPA accumulation was first clearly visible on a subset of axial elements in late zygotene spermatocytes, in which asynapsed chromosomes are first detected and marked by ATR-dependent H2AFX phosphorylation, and was coincident with BRCA1 (Fig. 1A,B; supplementary material Figs S1 and S2). As meiosis progresses into the pachytene stage of the first meiotic prophase, formation of the XY body and associated transcriptional repression is complete. At this stage, pRPA was enriched on sex chromosomes of all pachytene spermatocytes, most intensely at the axial element (Fig. 1C,D). In agreement with these immunofluorescence observations, western analysis of extracts from testes of postnatal mice showed a dramatic increase at day 10, coincident with the appearance of zygotene spermatocytes (supplementary material Fig. S3).
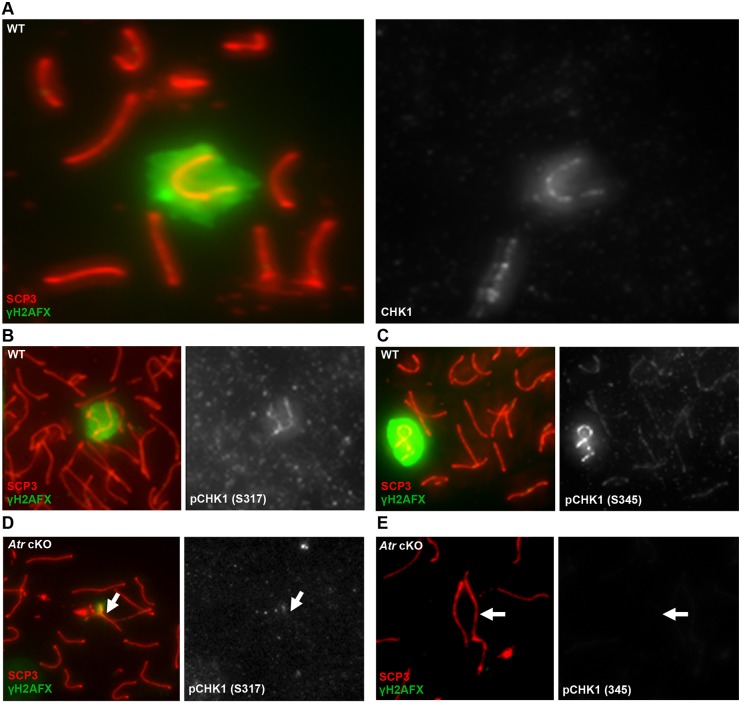
Fig. 1.
Phosphorylated RPA is a mark of asynapsed chromosomes in mouse primary spermatocytes. (A) RPA32 phosphorylated at serine 33 (pRPA) first colocalizes with the γH2AFX -coated sex chromosomes of wild-type (WT) late zygotene spermatocytes (arrows). (B) Higher magnification of the boxed region in A. (C) The sex chromosomes of all pachytene spermatocytes are enriched for pRPA. (D) Higher magnification view of XY body from C. pRPA staining is highest near axial elements (B,D, arrows). (E) An unpaired autosome in the XY body of Ts65(16:17)Dn spermatocytes, marked by BRCA1 and γH2AFX, is also enriched for pRPA. The arrow indicates the autosomal translocation.
To determine whether pRPA is a mark of the general MSUC pathway, we examined its localization in spermatocytes carrying an autosomal translocation between chromosomes 16 and 17 [Ts(16:17)65Dn (Epstein et al., 1985; Davisson et al., 1993)]. The presence of these duplicated chromosomal regions will manifest as an asynapsed autosome in the XY body of spermatocytes, as one copy (either the translocation or its homologous regions on the native chromosomes) will fail to pair (Turner et al., 2005; Reinholdt et al., 2009). In 74% of pachytene spermatocytes from Ts(16:17)65Dn males, a second axial element complex was present within the γH2AFX domain, in addition to the X and Y chromosomes. In all of these spermatocytes, both the sex chromosomes and the asynapsed autosome were enriched for pRPA (Fig. 1E). Together, these data suggest that the phosphorylation of serine 33 of RPA32 is a novel component of the MSUC pathway.
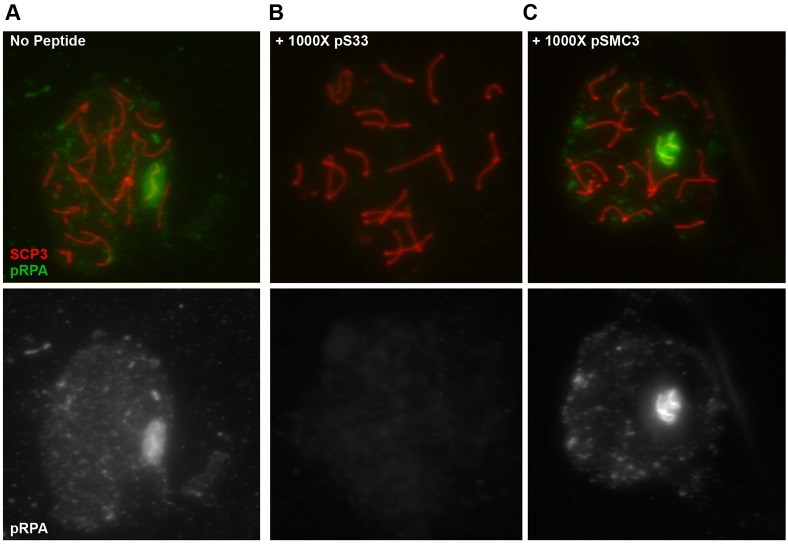
The axial element of the sex chromosomes is associated with numerous phosphoproteins, and axial element proteins of asynapsed chromosomes are themselves highly phosphorylated (Fukuda et al., 2012). Although this pRPA antibody has been validated in other studies (Shiotani et al., 2013), the high concentration of phosphoepitopes on the sex chromosomes might nonetheless contribute to non-specific binding. Therefore, we assessed the specificity of the pRPA antibody for immunofluorescence of spermatocyte spreads through competition with specific and non-specific phosphopeptides. Pre-incubation of the pRPA antibody with its specific phosphopeptide reduced the pRPA signal in pachytene spermatocytes, even at equal molar ratios between peptide and antibody (Fig. 2A,B; supplementary material Fig. S4). To determine whether a non-specific peptide could block the pRPA antibody, we utilized a phosphopeptide corresponding to structural maintenance of chromosomes 3 (SMC3), which is a subunit of the cohesion complex. Serine 1083 (S1083) of SMC3 is a target of the ATR/ATM kinases, and this phosphorylated form of SMC3 (pSMC3) is enriched on the axial element of the meiotic sex chromosomes (Fukuda et al., 2012). Since S1083 is within a target motif for ATR phosphorylation, and the spatial enrichment of pSMC3 corresponds to where we observe the highest pRPA signal, we speculated that the presence of this epitope might contribute to non-specific signal detection by the pRPA antibody. However, the pSMC3 peptide was unable to compete with the pRPA signal on the XY body even at a 1000-fold molar excess of peptide to antibody (Fig. 2C).
Fig. 2.
Specificity of pRPA antibody. WT spermatocytes were incubated with a molar excess of specific or non-specific phosphopeptides, along with antibodies against pRPA. The pRPA signal associated with the XY body (A) was completely abolished in the presence of a peptide specific for this antibody (pS33) (B). By contrast, even a 1000-fold molar excess of a peptide corresponding to SMC3, a known component of the XY body, had no effect on the association of the pRPA antibody with the sex chromosomes (C).
ATR is required for the enrichment of pRPA on meiotic sex chromosomes
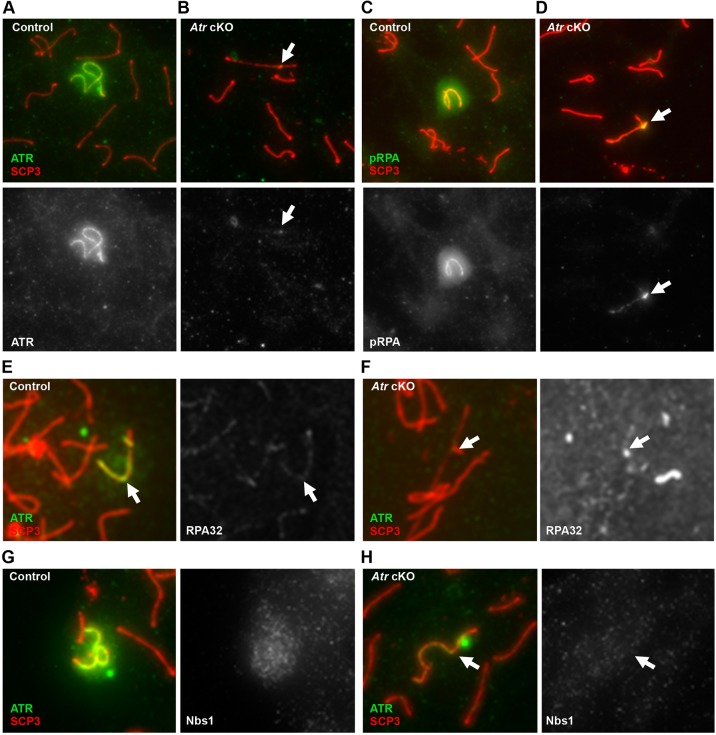
To confirm that the phosphorylation of RPA is related to ATR recruitment, we examined the distribution of pRPA in spermatocytes depleted of ATR. We utilized a strategy similar to that of Royo et al., inducing deletion of floxed Atr alleles by a tamoxifen-inducible Cre construct (Royo et al., 2013). As controls, age-matched Atrfl/fl males were treated with a similar course of tamoxifen. We recapitulated the published effects of ATR and γH2AFX distribution in spermatocytes from tamoxifen-treated Atrfl/fl; CAGG-CreERT2/0 males (referred to as Atr cKO). In these spermatocytes, the remaining ATR is associated with the pseudoautosomal region of the sex chromosomes (Fig. 3A,B, arrows). Importantly, we found that pRPA enrichment on the sex chromosomes was diminished in Atr cKO spermatocytes, and consistent with the lower levels and altered distribution of ATR (Fig. 3C,D). Immunostaining of spermatocytes from control and Atr cKO males with an antibody that detects RPA32 regardless of phosphorylation state revealed similar patterns of focal enrichment along the axial elements in both genotypes (Fig. 3E,F), suggesting that the majority of the RPA32 signal is independent of ATR. Thus, the RPA associated with the meiotic sex chromosomes is subject to ATR-dependent phosphorylation.
Fig. 3.
Phosphorylation of RPA on the meiotic sex chromosomes is dependent on ATR. ATR protein levels in spermatocytes from control (A) and Atr cKO (B) males. pRPA enrichment on the XY body in control (C) and Atr cKO (D) spermatocytes. Immunostaining with antibodies against RPA32, a component of the RPA complex, shows similar focal patterns in control pachytene spermatocytes (E) and spermatocytes from Atr cKO males with undetectable ATR protein (F). In control spermatocytes, the NBS1 nuclease is associated with the XY body (G). By contrast, Atr cKO spermatocytes with low levels of ATR had undetectable NBS1 enrichment on these chromosomes (H). Arrows indicate the sex chromosomes.
RPA32 can also be phosphorylated by the ataxia telangiectasia mutated (ATM) kinase, which is responsible for H2AFX phosphorylation in response to DSBs. Therefore, we examined the distribution of pRPA in Atm−/− spermatocytes. These mutant spermatocytes show significant pairing defects and arrest during early pachynema (Xu et al., 1996; Barlow et al., 1998; Barchi et al., 2005). However, pRPA remained associated with γH2AFX-coated axial elements in Atm−/− spermatocytes (supplementary material Fig. S5A,B). These results confirm that ATR is the primary DNA damage kinase responsible for the phosphorylation of RPA32 on the XY body.
The accumulation and subsequent ATR-dependent phosphorylation of RPA at damaged replication forks in somatic cells is dependent on the MRN (MRE11-RAD50-NBS1) nuclease complex (Shiotani et al., 2013). The MRN complex catalyzes exonucleolytic processing of these lesions to produce long stretches of ssDNA/RPA. This accumulation of ssDNA/RPA recruits additional ATR, which in turn phosphorylates RPA. Interestingly, components of the MRN complex are known to associate with the XY, and in the case of NBS1 (NBN – Mouse Genome Informatics) this association is independent of SPO11 (Barchi et al., 2005). However, the function of NBS1 and the MRN complex during MSCI has remained undefined. We speculated that the accumulation of pRPA on the XY body is linked to a similar processing mechanism, whereby ATR promotes MRN localization to the sex chromosomes to generate additional ssDNA/RPA. Thus, spermatocytes depleted for ATR should show a decrease in MRN enrichment on the XY. We examined ATR-depleted spermatocytes for the distribution of NBS1, the MRN component with the most pronounced role in promoting pRPA accumulation in replicating somatic cells (Shiotani et al., 2013). In spermatocytes from wild-type animals, NBS1 was frequently associated with the XY, and we confirmed this association was SPO11 independent (Fig. 3G; supplementary material Fig. S6). By contrast, NBS1 accumulation on the sex chromosomes was not observed in spermatocytes from Atr cKO males with undetectable levels of ATR (Fig. 3H). These data demonstrate that recruitment of NBS1 to the XY is not related to the processing of SPO11-dependent DSBs, but instead is elicited by ATR recruitment. However, the localization of NBS1 on the sex chromosomes is more diffuse than that of pRPA, suggesting that the MRN complex might have additional roles in MSCI, perhaps underlying the foci of the ssDNA-binding protein RAD51 observed throughout the meiotic sex chromosomes (Plug et al., 1998). Together with the presence of pRPA, these observations suggest that the sex chromosomes might undergo a DNA-processing event as part of MSCI, linked to ATR recruitment.
pRPA is associated with asynapsed chromatin in the absence of SPO11-dependent meiotic DSBs
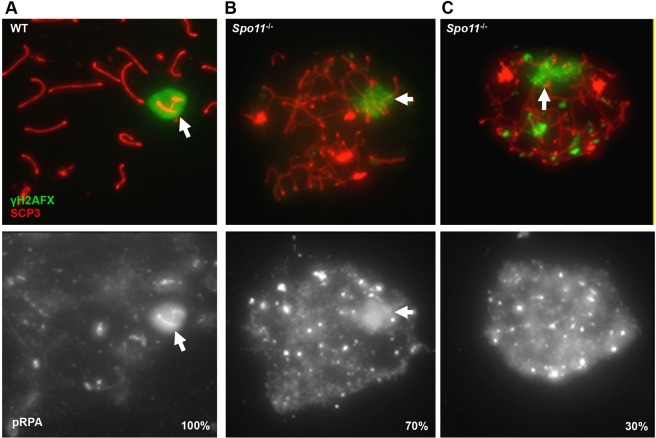
SPO11 is an evolutionarily conserved enzyme responsible for generating the programmed DSBs during meiosis. Because DSBs are required for successful synapsis, Spo11−/− spermatocytes are characterized by significant numbers of asynapsed chromosomes and fail to complete the first meiotic prophase (Baudat et al., 2000; Romanienko and Camerini-Otero, 2000). However, ATR enrichment and coincident transcriptional repression still occur, suggesting MSUC can be initiated in Spo11−/− spermatocytes (Mahadevaiah et al., 2008). Thus, if pRPA represents regions of meiotic ATR activation (rather than a processing event of DSBs on asynapsed chromosomes), then it should remain coincident with γH2AFX-coated chromosomes even in the absence of SPO11-dependent meiotic DSBs. In agreement with this prediction, 70% (n=189) of Spo11−/− spermatocytes with a single, clearly defined domain of γH2AFX had coincident enrichment of pRPA (Fig. 4A,B). In the remaining 30% of Spo11−/− spermatocytes, pRPA was present, yet more broadly distributed than γH2AFX (Fig. 4C). Therefore, these observations demonstrate that the phosphorylation of RPA occurs in Spo11−/− spermatocytes and suggests that the ssDNA to which this RPA is bound is generated independently of the SPO11-dependent mechanism of DSB formation.
Fig. 4.
Phosphorylation of RPA is independent of Spo11. (A) pRPA is associated with the XY chromosomes in 100% of WT spermatocytes. (B) 70% of Spo11−/− spermatocytes show colocalization of pRPA with γH2AFX-positive domains. (C) Broad, nuclear pRPA distribution is evident in 30% of mutant spermatocytes. Bright pRPA foci are common in both classes of Spo11−/− spermatocytes. Arrows indicate regions undergoing MSUC.
Phosphorylated CHK1 associates with asynapsed chromatin
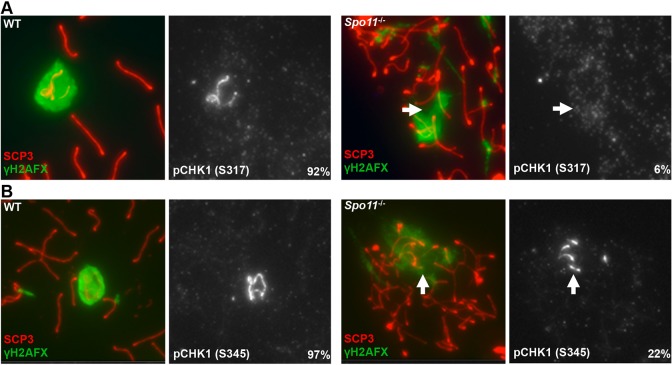
The accumulation of ssDNA/RPA during replication stress activates pathways to repair stalled replication forks, as well as a checkpoint pathway to elicit physiological responses such as control of the cell cycle. This latter function is mediated through the ATR-dependent phosphorylation of CHK1, a checkpoint protein and potent physiological effector of ATR activity (Liu et al., 2000). Although CHK1 has previously been shown to associate with all axial elements in pachytene spermatocytes (Flaggs et al., 1997), we observed that CHK1 was highly enriched on the sex chromosomes (Fig. 5A). Furthermore, CHK1 phosphorylated at the ATR target residues serines 317 (S317) and 345 (S345) was detectable near the axial element of the sex chromosomes from late zygotene onwards (Fig. 5B,C; supplementary material Fig. S7A,B). Consistent with these residues of CHK1 being direct targets of ATR kinase activity, phosphorylated CHK1 (pCHK1) was not observed in spermatocytes depleted for ATR (Fig. 5D,E). In addition, pCHK1 accumulated on an asynapsed autosomal translocation and γH2AFX-coated axial elements of Atm−/− spermatocytes outside the XY body, demonstrating pCHK1 as a marker of asynapsed chromatin (supplementary material Fig. S8). However, this pattern of enrichment was abrogated in Spo11−/− spermatocytes, despite pervasive asynapsis. Only 6% (n=47) and 22% (n=89) of mutant spermatocytes with a single, large domain of γH2AFX (identifying regions undergoing MSUC) had detectable colocalization of phosphorylated S317 and S345, respectively (Fig. 6A,B). Thus, in contrast to pRPA, the stable association of pCHK1 with asynapsed chromosomes is strongly dependent on SPO11.
Fig. 5.
ATR-dependent localization and phosphorylation of CHK1 on the XY body. The asynapsed sex chromosomes show enrichment of the checkpoint protein CHK1 in WT spermatocytes (A). Furthermore, the XY body of WT spermatocytes is associated with phosphorylated forms of CHK1: at serine 317 (S317; B) and serine 345 (S345; C), both of which are target residues of the ATR kinase. The enrichment of pCHK1 (S317) and pCHK1 (S345) is undetectable in spermatocytes from Atr cKO males (D,E). Arrows indicate the position of the XY chromosomes.
Fig. 6.
Spo11 deficiency severely abrogates the association of pCHK1 with asynapsed chromatin. Localization of pCHK1 (S317) (A) and pCHK1 (S345) (B) to the sex chromosomes and regions undergoing MSUC in WT and Spo11−/− spermatocytes. Arrows indicate regions undergoing MSUC.
DISCUSSION
Activation of MSCI results in chromosome-wide transcriptional repression, a silent state that is maintained during the subsequent meiotic divisions (Turner, 2007). Although the ATR DNA damage response kinase plays an essential role in the initiation of this epigenetic phenomenon, the molecular signals that distinguish an asynapsed chromosome and recruit ATR to these genomic regions in primary spermatocytes have remained the subject of speculation. Recent observations of RPA foci in Spo11−/− spermatocytes, in which the MSUC pathway is still active, are indicative of ssDNA in meiotic genomes (Carofiglio et al., 2013). Given the crucial role for RPA/ssDNA in stimulating ATR activation in somatic cells, it has been proposed that a similar mechanism may contribute to the recruitment and activation of ATR on asynapsed chromatin (Ichijima et al., 2012). Here, we demonstrate that asynapsed chromosomes are enriched for pRPA in an ATR-dependent, but SPO11-independent, manner. This enrichment is temporally coincident with MSCI activation, and the localization of pRPA near the axial elements of asynapsed chromosomes is spatially coincident with other factors involved in the early stages of MSCI. Since RPA is highly phosphorylated during ATR activation in somatic cells, marking genomic sites of DNA damage, we propose that regions of the sex chromosomes where pRPA accumulates would mark the DNA elements at which the MSUC/MSCI response is initiated. These elements might act as sensors of pairing, becoming repaired or resolved into double-stranded DNA upon successful synapsis, but persisting as ssDNA where synapsis fails into late zygonema, when they serve as platforms for the activation of MSUC.
Like ATR, the DNA repair protein BRCA1 is an essential component of MSCI/MSUC activation, and one of the earliest cytological markers of asynapsed chromosomes. Although the best-characterized role of BRCA1 in somatic DNA damage responses is in promoting the repair of DSBs by homologous recombination, its function during MSCI/MSUC appears distinct from the recognition or repair of SPO11-dependent meiotic DSBs (Mahadevaiah et al., 2008; Broering et al., 2014). Therefore, how BRCA1 participates in the detection of asynapsed chromatin to trigger ATR recruitment remains unknown. Interestingly, BRCA1 promotes ATR activation in response to UV-induced DNA damage in somatic cells (Pathania et al., 2011). UV irradiation results in the formation of bulky, covalent adducts to DNA, which impede or block the progression of replication forks. At adduct-stalled replication forks, BRCA1 is required for the formation of long stretches of ssDNA which, when coated with RPA, will recruit and activate ATR. Thus, BRCA1 might serve a similar role during the early stages of MSCI/MSUC, increasing the size of SPO11-independent, RPA-coated ssDNA regions on asynapsed chromosomes to elicit high levels of ATR activation. Notably, although ATR activation is severely affected in the majority of Brca1 mutant spermatocytes, it is not entirely ablated, suggesting that some ssDNA/RPA regions are still present and may produce the limited ATR response observed in the absence of BRCA1.
It is not yet clear whether regions of SPO11-independent RPA are a product of the DNA replication that precedes meiotic entry or a replication-independent process that is coordinated with the events of synapsis. Interestingly, a limited amount of replicative DNA synthesis has been reported to occur during zygonema, although the significance of this observation has remained enigmatic (Stern and Hotta, 1984; Hotta et al., 1985; Stubbs and Stern, 1986). In somatic cells, the most robust phosphorylation of RPA occurs at replication-induced DSBs (Shiotani et al., 2013). These lesions are formed as the replication fork encounters a single-stranded break in the DNA strand, producing a ‘single-ended’ DSB. In this context, ATR promotes the recruitment of the MRN complex (including NBS1) to generate extensive ssDNA/RPA by resecting this DSB. Similarly, the ATR-dependent recruitment of NBS1 to the XY chromosomes suggest that an ATR-dependent processing event is a component of MSCI. However, it is also possible that an ssDNA structure similar to that of a stalled replication fork might be generated independently of replicative DNA synthesis. For example, transcription may be a source of DNA damage (Sordet et al., 2009). Interestingly, the XY body is enriched for senataxin, a protein that functions in the resolution of RNA-DNA hybrids (R-loops) formed during transcriptional elongation (Becherel et al., 2013). If DNA lesions brought about by transcription were to play a role in the activation of the MSUC pathway, they would have to be formed in the early stages of meiotic prophase since asynapsed regions are transcriptionally silenced. Regardless of their source, our observations provide support for a model whereby regions of ssDNA or ssDNA-bearing secondary structures are likely to play an important role in the activation of MSUC.
In addition to pRPA, we found that the XY chromosomes were enriched for active, phosphorylated forms of the checkpoint protein CHK1, in an ATR-dependent manner. A previous report showed CHK1 broadly associated with the synaptonemal complexes of all chromosomes in mouse pachytene spermatocytes (Flaggs et al., 1997). The discrepancy with our observations might be related to the different antisera used. However, given the known dependence of CHK1 activation on ATR in somatic DNA damage pathways, and the enrichment of ATR on the XY body, we believe it is more likely that CHK1 is highly, if not exclusively, enriched on asynapsed chromosomes and is a component of the MSUC pathway. Yet, in contrast to the localization of pRPA, enrichment of pCHK1 to asynapsed chromosomes was severely compromised in Spo11−/− spermatocytes. Therefore, the physical association of CHK1 with asynapsed chromatin might be dependent upon SPO11-induced DSBs, or perhaps antagonized by the increased ATR activation brought about by excessive asynapsis in these mutant spermatocytes. Interestingly, the shuttling of CHK1 in and out of the nucleus has been observed in somatic cells, and this was speculated as a mechanism by which CHK1 attenuates cell-cycle progression in response to DNA damage signaling (Smits et al., 2006; Wang et al., 2012). An analogous event might take place in Spo11−/− spermatocytes, where the extent of asynapsis crosses a critical threshold to elicit the nuclear export of CHK1.
Collectively, these data suggest that CHK1 may function as a molecular link between ATR signaling at asynapsed chromosomes and meiotic progression, and reveal additional similarities between somatic ATR activation and MSCI/MSUC.
MATERIALS AND METHODS
Mouse strains and animal work
Atmtm1Awb, Spo11tm1Mjn, Ts65(16:17)Dn and B6.Cg-Tg(CAG-Cre/Esr1)5Amc/J (CAGG-CreERT2) mice were obtained from Jackson Laboratories. Mice carrying a conditional allele of Atr have been described previously (Ruzankina et al., 2007). Genotyping for all alleles was performed as previously described (Ruzankina et al., 2007). To delete the floxed Atr allele, 5- to 8-week-old Atrfl/fl; CAGG-CreERT2/0 males were treated with 4 mg tamoxifen (Sigma) in two to four doses at 48 h intervals by intraperitoneal injection, and spermatocytes were harvested 10 days after the first treatment. All mouse work was performed in accordance with IACUC protocols at UNC.
Spermatocyte spreads
Spermatocyte spreads were prepared as described (Peters et al., 1997), except that slides were washed once for 5 min at room temperature in 0.32% Photoflo (Kodak) in 1×PBS after fixing. Slides were air dried and stored at −80°C until use.
Immunofluorescence
Slides were washed for 10 min each at room temperature in: 0.32% Photoflo in 1×PBS; 0.1% Triton X-100 in 1×PBS; and 0.3% BSA (Fisher), 1% donkey serum (Jackson ImmunoResearch), 0.015% Triton X-100 in 1×PBS. All primary antibodies were diluted in ADB buffer (3% BSA, 10% donkey serum, 0.05% Triton X-100 in 1×PBS) and incubated overnight at 4°C, with the exception of RPA32 antibody which was incubated for 1 h at 37°C. After washing, slides were incubated for 1 h at room temperature with secondary antibodies diluted in ADB buffer. Slides were then washed three times for 5 min each in 0.32% Photoflo in 1×PBS. To detect γH2AFX, slides were then fixed for 10 min at room temperature with 4% paraformaldehyde (Electron Microscopy Sciences) in 1×PBS, washed extensively with 0.32% Photoflo in 1×PBS, then incubated for 1-4 h at room temperature with mouse anti-γH2AFX (Millipore, 05-636), which was directly labeled using the Alexa Fluor 488 Microscale Protein Labeling Kit according to manufacturer's instructions (Invitrogen) and diluted 1:200 in ADB before use. After three 5 min washes in 0.32% Photoflo in 1×PBS, and one wash for 5 min with 0.32% Photoflo in double-distilled water, slides were mounted with SlowFade Gold (Invitrogen). z-stacks of each channel were taken on a Zeiss AxioImager M2 microscope, using the Axiovision software package (Zeiss). SCP3 and γH2AFX patterns were used to stage spermatocytes, and intensity was adjusted for presentation purposes. Experiments were carried out in two or three animals per genotype and antibody. For peptide competition experiments, varying amounts of each peptide (Bethyl) were pre-incubated with the antibody for 30 min at room temperature in ADB buffer prior to immunofluorescence.
Antibodies
The following primary antibodies (and dilutions) were used for immunofluorescence: rabbit anti-phosphoRPA32, serine 33 (Bethyl, A300-246A; 1:200); rabbit anti-RPA32 (Bethyl, A300-244A; 1:100); mouse anti-SCP3 (Abcam, ab97672; 1:500); mouse anti-BRCA1 (a gift from David Livingston, Harvard Medical School; 1:50); rabbit anti-phosphoCHK1, serine 317 (Cell Signaling, 2344S; 1:25); rabbit anti-phosphoCHK1, serine 345 (Cell Signaling, 2341S; 1:50); rabbit anti-CHK1 (Santa Cruz, sc7898; 1:25); rabbit anti-ATR (Calbiochem, PC538; 1:400); and mouse anti-NBS1 (BD Biosciences, 611870; 1:10). The secondary antibodies goat anti-rabbit Alexa647, goat anti-mouse Alexa568, goat anti-rabbit Alexa568 and goat anti-mouse Alexa488 (all Invitrogen, A-20991, A-11031, A-11036 and A-11029, respectively) were diluted 1:500. DNA was counterstained with DAPI. Phospho-specific antibodies did not produce a signal when tested against phosphatase-treated spermatocyte spreads.
Supplementary Material
Acknowledgements
We thank Drs Cyrus Vaziri, Joshua Starmer and members of the T.M. Laboratory for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.M.F. conceived, designed and performed the experiments with input from T.M., D.M., Y.K. and W.M. performed the experiments. A.M.F. and T.M. prepared the manuscript. All authors commented on the manuscript.
Funding
This work was supported by grants from the National Institutes of Health [1F32HD052413 to A.M.F., R01-GM10974 and DPU-U01-14817 to T.M.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.126078/-/DC1
References
- Anantha R. W., Vassin V. M. and Borowiec J. A. (2007). Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair. J. Biol. Chem. 282, 35910-35923. 10.1074/jbc.M704645200 [DOI] [PubMed] [Google Scholar]
- Barchi M., Mahadevaiah S., Di Giacomo M., Baudat F., de Rooij D. G., Burgoyne P. S., Jasin M. and Keeney S. (2005). Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol. Cell. Biol. 25, 7203-7215. 10.1128/MCB.25.16.7203-7215.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C., Liyanage M., Moens P. B., Tarsounas M., Nagashima K., Brown K., Rottinghaus S., Jackson S. P., Tagle D., Ried T. et al. (1998). Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 125, 4007-4017. [DOI] [PubMed] [Google Scholar]
- Baudat F., Manova K., Yuen J. P., Jasin M. and Keeney S. (2000). Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 6, 989-998. 10.1016/S1097-2765(00)00098-8 [DOI] [PubMed] [Google Scholar]
- Becherel O. J., Yeo A. J., Stellati A., Heng E. Y. H., Luff J., Suraweera A. M., Woods R., Fleming J., Carrie D., McKinney K. et al. (2013). Senataxin plays an essential role with DNA damage response proteins in meiotic recombination and gene silencing. PLoS Genet. 9, e1003435 10.1371/journal.pgen.1003435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani M. A., Romanienko P. J., Cairatti D. A. and Camerini-Otero R. D. (2005). SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm-/- spermatocytes. J. Cell Sci. 118, 3233-3245. 10.1242/jcs.02466 [DOI] [PubMed] [Google Scholar]
- Broering T. J., Alavattam K. G., Sadreyev R. I., Ichijima Y., Kato Y., Hasegawa K., Camerini-Otero R. D., Lee J. T., Andreassen P. R. and Namekawa S. H. (2014). BRCA1 establishes DNA damage signaling and pericentric heterochromatin of the X chromosome in male meiosis. J. Cell Biol. 205, 663-675. 10.1083/jcb.201311050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carofiglio F., Inagaki A., de Vries S., Wassenaar E., Schoenmakers S., Vermeulen C., van Cappellen W. A., Sleddens-Linkels E., Grootegoed J. A., te Riele H. P. J. et al. (2013). SPO11-independent DNA repair foci and their role in meiotic silencing. PLoS Genet. 9, e1003538 10.1371/journal.pgen.1003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davisson M. T., Schmidt C., Reeves R. H., Irving N. G., Akeson E. C., Harris B. S. and Bronson R. T. (1993). Segmental trisomy as a mouse model for Down syndrome. Prog. Clin. Biol. Res. 384, 117-133. [PubMed] [Google Scholar]
- Epstein C. J., Cox D. R. and Epstein L. B. (1985). Mouse trisomy 16: an animal model of human trisomy 21 (Down syndrome). Ann. N. Y. Acad. Sci. 450, 157-168. 10.1111/j.1749-6632.1985.tb21490.x [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O., Mahadevaiah S. K., Celeste A., Romanienko P. J., Camerini-Otero R. D., Bonner W. M., Manova K., Burgoyne P. and Nussenzweig A. (2003). H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev. Cell 4, 497-508. 10.1016/S1534-5807(03)00093-5 [DOI] [PubMed] [Google Scholar]
- Flaggs G., Plug A. W., Dunks K. M., Mundt K. E., Ford J. C., Quiggle M. R. E., Taylor E. M., Westphal C. H., Ashley T., Hoekstra M. F. et al. (1997). Atm-dependent interactions of a mammalian chk1 homolog with meiotic chromosomes. Curr. Biol. 7, 977-986. 10.1016/S0960-9822(06)00417-9 [DOI] [PubMed] [Google Scholar]
- Fukuda T., Pratto F., Schimenti J. C., Turner J. M. A., Camerini-Otero R. D. and Hoog C. (2012). Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet. 8, e1002485 10.1371/journal.pgen.1002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Tabata S., Stubbs L. and Stern H. (1985). Meiosis-specific transcripts of a DNA component replicated during chromosome pairing: homology across the phylogenetic spectrum. Cell 40, 785-793. 10.1016/0092-8674(85)90338-1 [DOI] [PubMed] [Google Scholar]
- Ichijima Y., Ichijima M., Lou Z., Nussenzweig A., Camerini-Otero R. D., Chen J., Andreassen P. R. and Namekawa S. H. (2011). MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev. 25, 959-971. 10.1101/gad.2030811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijima Y., Sin H.-S. and Namekawa S. H. (2012). Sex chromosome inactivation in germ cells: emerging roles of DNA damage response pathways. Cell. Mol. Life Sci. 69, 2559-2572. 10.1007/s00018-012-0941-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Guntuku S., Cui X. S., Matsuoka S., Cortez D., Tamai K., Luo G., Carattini-Rivera S., DeMayo F., Bradley A. et al. (2000). Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14, 1448-1459. [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah S. K., Bourc'his D., de Rooij D. G., Bestor T. H., Turner J. M. A. and Burgoyne P. S. (2008). Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J. Cell Biol. 182, 263-276. 10.1083/jcb.200710195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania S., Nguyen J., Hill S. J., Scully R., Adelmant G. O., Marto J. A., Feunteun J. and Livingston D. M. (2011). BRCA1 is required for postreplication repair after UV-induced DNA damage. Mol. Cell 44, 235-251. 10.1016/j.molcel.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. H. F. M., Plug A. W., van Vugt M. J. and de Boer P. (1997). A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5, 66-68. 10.1023/A:1018445520117 [DOI] [PubMed] [Google Scholar]
- Plug A. W., Peters A. H., Keegan K. S., Hoekstra M. F. and de Boer P. (1998). Changes in protein composition of meiotic nodules during mammalian meiosis. J. Cell Sci. 111, 412-423. [DOI] [PubMed] [Google Scholar]
- Reinholdt L. G., Czechanski A., Kamdar S., King B. L., Sun F. and Handel M. A. (2009). Meiotic behavior of aneuploid chromatin in mouse models of Down syndrome. Chromosoma 118, 723-736. 10.1007/s00412-009-0230-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanienko P. J. and Camerini-Otero R. D. (2000). The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 6, 975-987. 10.1016/S1097-2765(00)00097-6 [DOI] [PubMed] [Google Scholar]
- Royo H., Prosser H., Ruzankina Y., Mahadevaiah S. K., Cloutier J. M., Baumann M., Fukuda T., Hoog C., Toth A., de Rooij D. G. et al. (2013). ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev. 27, 1484-1494. 10.1101/gad.219477.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V. P., Velez M., Bhandoola A. and Brown E. J. (2007). Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113-126. 10.1016/j.stem.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimenti J. (2005). Synapsis or silence. Nat. Genet. 37, 11-13. 10.1038/ng0105-11 [DOI] [PubMed] [Google Scholar]
- Shiotani B., Nguyen H. D., Hakansson P., Marechal A., Tse A., Tahara H. and Zou L. (2013). Two distinct modes of ATR activation orchestrated by Rad17 and Nbs1. Cell Rep. 3, 1651-1662. 10.1016/j.celrep.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits V. A. J., Reaper P. M. and Jackson S. P. (2006). Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr. Biol. 16, 150-159. 10.1016/j.cub.2005.11.066 [DOI] [PubMed] [Google Scholar]
- Sordet O., Redon C. E., Guirouilh-Barbat J., Smith S., Solier S., Douarre C., Conti C., Nakamura A. J., Das B. B., Nicolas E. et al. (2009). Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 10, 887-893. 10.1038/embor.2009.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern H. and Hotta Y. (1984). Chromosome organization in the regulation of meiotic prophase. Symp. Soc. Exp. Biol. 38, 161-175. [PubMed] [Google Scholar]
- Stubbs L. and Stern H. (1986). DNA synthesis at selective sites during pachytene in mouse spermatocytes. Chromosoma 93, 529-536. 10.1007/BF00386795 [DOI] [PubMed] [Google Scholar]
- Turner J. M. A. (2007). Meiotic sex chromosome inactivation. Development 134, 1823-1831. 10.1242/dev.000018 [DOI] [PubMed] [Google Scholar]
- Turner J. M. A., Aprelikova O., Xu X., Wang R., Kim S., Chandramouli G. V. R., Barrett J. C., Burgoyne P. S. and Deng C.-X. (2004). BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 14, 2135-2142. 10.1016/j.cub.2004.11.032 [DOI] [PubMed] [Google Scholar]
- Turner J. M., Mahadevaiah S. K., Fernandez-Capetillo O., Nussenzweig A., Xu X., Deng C. X. and Burgoyne P. S. (2005). Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37, 41-47. [DOI] [PubMed] [Google Scholar]
- Vassin V. M., Anantha R. W., Sokolova E., Kanner S. and Borowiec J. A. (2009). Human RPA phosphorylation by ATR stimulates DNA synthesis and prevents ssDNA accumulation during DNA-replication stress. J. Cell Sci. 122, 4070-4080. 10.1242/jcs.053702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Han X., Feng X., Wang Z. and Zhang Y. (2012). Coupling cellular localization and function of checkpoint kinase 1 (Chk1) in checkpoints and cell viability. J. Biol. Chem. 287, 25501-25509. 10.1074/jbc.M112.350397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Ashley T., Brainerd E. E., Bronson R. T., Meyn M. S. and Baltimore D. (1996). Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10, 2411-2422. 10.1101/gad.10.19.2411 [DOI] [PubMed] [Google Scholar]
- Zou L. and Elledge S. J. (2003). Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542-1548. 10.1126/science.1083430 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.