Fig. 7.
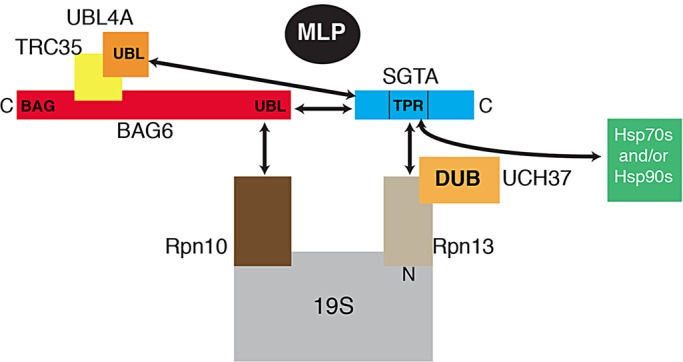
Model describing the modulation of MLP quality control at the proteasome by BAG6 and SGTA. The heterotrimeric BAG6 complex, composed of Bag6, TRC35 and UBL4A, recognises cytosolic MLPs and stimulates their ubiquitylation. It might also contribute to substrate delivery to the proteasome following the interaction of the Bag6 subunit with Rpn10 (Kikukawa et al., 2005; Minami et al., 2010). SGTA is recruited to the C-terminal region of Rpn13 through its central TPR domain (this study), in concert with MLPs that might bind to SGTA and/or the Pru domain of Rpn13, depending on their ubiquitylation status. The proteasome-associated deubiquitylase UCH37 also binds Rpn13 (Bhattacharyya et al., 2014; Hamazaki et al., 2006; Yao et al., 2006), providing a potential molecular basis for a putative ‘rescue pathway’ that facilitates the deubiquitylation of previously modified MLPs (Leznicki and High, 2012; Wunderley et al., 2014). The proximity of the Rpn10 and Rpn13 subunits (Bhattacharyya et al., 2014) is consistent with the suggestion that, following delivery to the proteasome, substrates undergo cycles of ubiquitylation and deubiquitylation in response to the respective actions of the BAG6 complex and SGTA (Hessa et al., 2011; Leznicki and High, 2012; Rodrigo-Brenni et al., 2014; Wunderley et al., 2014). The Hsp70 and Hsp90 molecular chaperones could also contribute to this hypothetical quality-control process (Liou and Wang, 2005; Walczak et al., 2014).
