Fig. 1.
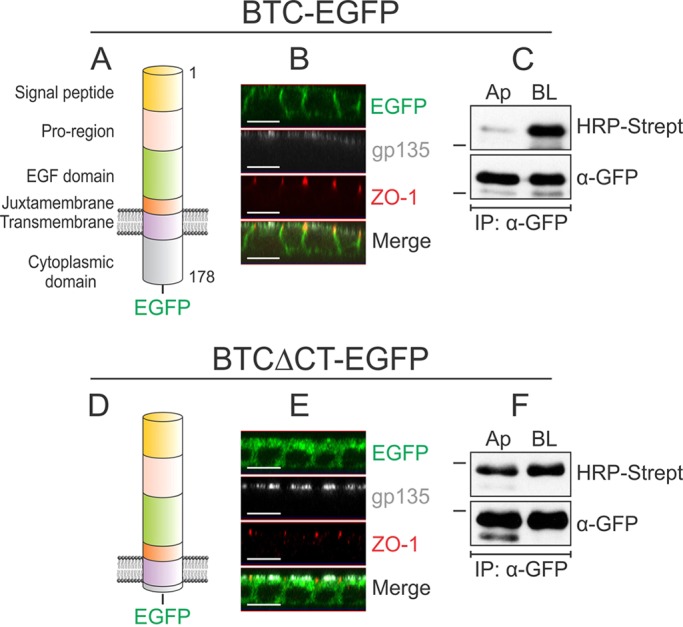
Localization of BTC to the basolateral surface depends on its cytoplasmic domain. (A) Domain organization of human BTC. The 178-amino-acid pre-propeptide contains a 31-amino-acid signal peptide, a 33-amino-acid pro-region, a 41-amino-acid EGF domain, a 13-amino-acid juxtamembrane domain, a 21-amino-acid transmembrane domain and a 39-amino-acid cytoplasmic domain. The soluble 80-amino-acid BTC (residues 32–111) comprises the pro-region, EGF domain and part of the juxtamembrane region. (B) MDCK cells stably expressing BTC–EGFP were grown on Transwell filters for 5 days and were then paraformaldehyde-fixed and immunostained for ZO-1 (red) and gp135 (white); BTC–EGFP fluorescence is shown in green. Confocal projections in the xz plane are shown. (C) Polarized MDCK cells stably expressing BTC–EGFP were subjected to selective cell-surface biotinylation on either the apical (Ap) or basolateral (BL) surfaces. Cells were then lysed and immunoprecipitated (IP) with an anti-GFP antibody (α-GFP), and proteins were resolved on SDS-PAGE gels and probed with HRP–streptavidin to measure the extent of biotinylation on each surface (upper panel). Reblotting for GFP (lower panel) confirmed equal loading. (D) A construct lacking the distal 35 amino acids of the 39-amino-acid cytoplasmic domain is designated BTCΔCT–EGFP. (E) MDCK cells stably expressing BTCΔCT–EGFP were processed as cells described in B and immunostained for ZO-1 (red) and gp135 (white). (F) MDCK cells stably expressing BTCΔCT–EGFP were subjected to selective cell-surface biotinylation and displayed as described in C. All experiments were performed at least three times; representative images and blots are shown here. Dashes on the left of western blots indicate a molecular mass of 55 kDa. Scale bars: 10 μm.
