Fig. 2.
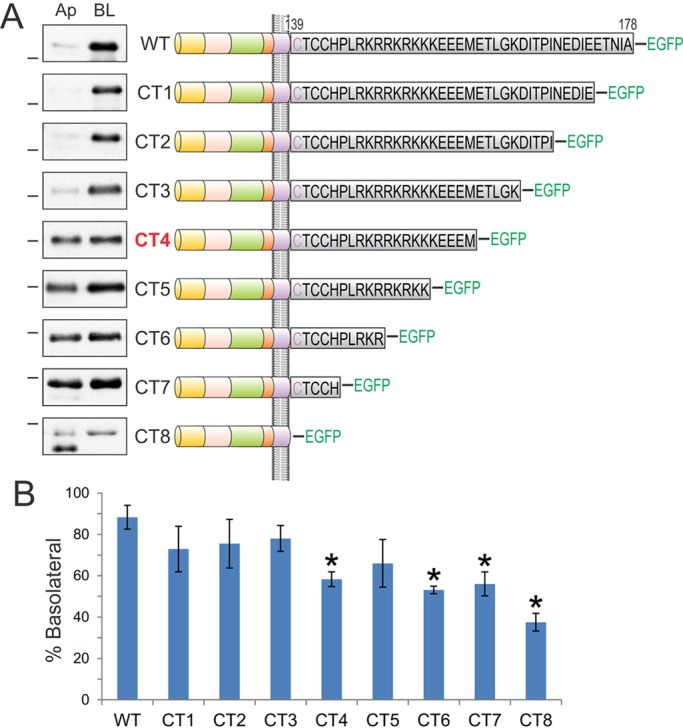
Analysis of sequential cytoplasmic domain truncations of BTC identifies its basolateral-sorting regions. (A) The BTC cytoplasmic domain is expanded to display the individual 39 amino acids in black. BTC is palmitoylated within the juxtamembrane CTCC motif; the first cysteine residue (depicted in purple) is predicted to reside within the transmembrane domain. Polarized MDCK cells stably expressing successive 5-amino-acid BTC truncations fused to EGFP at the C-terminus (CT1 to CT8) were subjected to selective cell-surface biotinylation on apical (Ap) or basalolateral (BL) cell surfaces. Note that the results for CT7 are shown as BTCΔCT–EGFP in Fig. 1F. Cellular lysates were harvested and subjected to GFP immunoprecipitation, and proteins were resolved on SDS-PAGE and then probed with HRP–streptavidin to measure the extent of biotinylation, which is displayed in the panel on the left. Dashes on the left of western blots indicate a molecular mass of 55 kDa. (B) Quantification of western blots. % Basolateral=([BL]/[Ap]+[BL])×100. Combined results from at least three independent experiments are represented as mean±s.e.m., *statistically significant difference (P<0.05, two-tailed unpaired t-test) compared with wild type.
