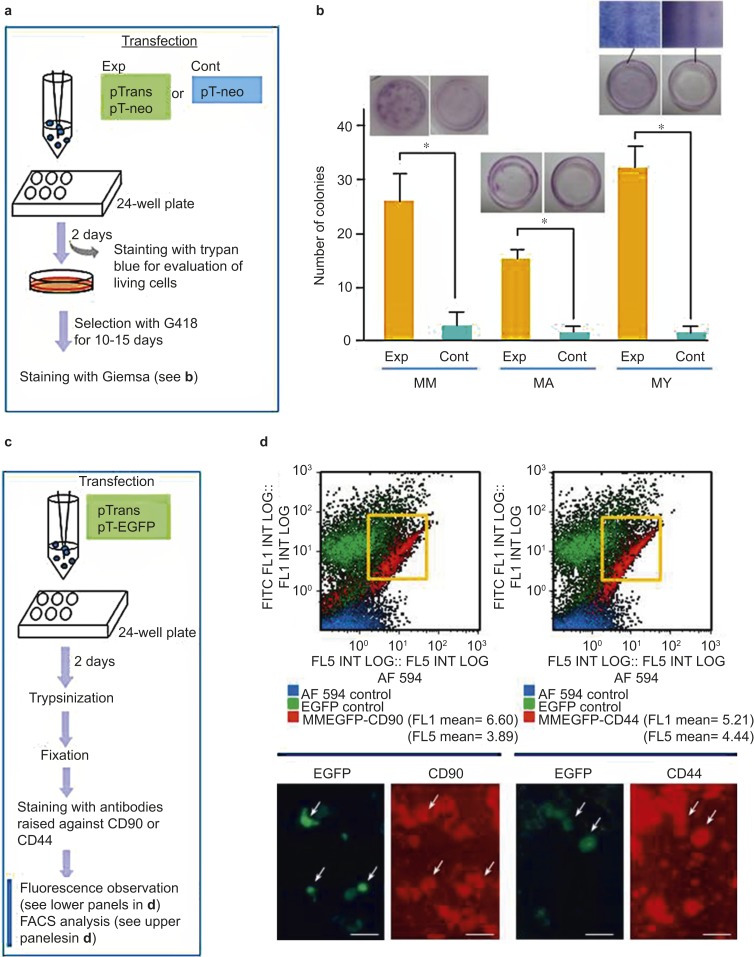
Figure 2.
Efficiency of the PB-based gene delivery system for acquisition of stable transfectants. (a) A flowchart of the experiments used to test the activity of PB transposase in HDDPCs established from three donors (MM, MA, and YY). pTrans was used as a positive control for PB transposase expression in the experimental group (Exp). In the control group (Cont), only pT-neo was used for transfection. After transfection, cells were plated onto a 24-well plates and then selected in the presence of G418 for 10–15 days until colonies emerged. The cells were fixed with 4% PFA and stained with Giemsa stain for colony counting. (b) Graphical representation of the number of colonies that survived after drug selection. The experiments were repeated three times, and the mean ± standard deviations of the number of colonies is shown above each bar. The photomicrographs above the bars show representative images of Giemsa-stained cells. A magnified view of the Giemsa-stained colonies is shown for YY. (c) A flowchart of the experiments used to test whether PB-based gene delivery permits transfection of certain specific populations of cells included in the HDDPCs. (d) Fluorescence-activated cell sorting (FACS) (upper panels) and EGFP fluorescence (lower panels) analyses of MM HDDPCs one day after transfection with pTrans and pT-EGFP and subsequent staining with antibodies for CD90 or CD44. The majority of HDDPCs positively stained for these antibodies, and of these, the transfected green fluorescent cells (arrows) are visible. Note that there is no appreciable deviation in the distribution of between EGFP- and CD44- or CD90-positive cells (shown in quadrants). CD, cluster of differentiation; EGFP, enhanced green fluorescent protein; HDDPC, human deciduous tooth dental pulp cell; PFA, paraformaldehyde.