Abstract
The present study evaluated the expression of key molecules and the status of DNA in both oral squamous cell carcinoma (OSCC) and adjacent tissues to establish a molecular surgical boundary and provide a cancer progression model. Biopsy samples from 50 OSCC patients were divided into T (cancer), P1 (0–0.5 cm), P2 (0.5–1 cm), P3 (1–1.5 cm) and P4 (1.5–2 cm) groups based on the distances from the visible boundary of the primary focus. Twenty samples of normal mucosa were used as controls. We used immunohistochemical staining and flow cytometry to evaluate p53, p21 CIP1/WAF1 , eIF4E and Ki-67 expression and to determine DNA status, respectively. Sub-mucosal invasion was present in the P1 and P2 groups as determined by haematoxylin and eosin staining. Mutant p53 expression decreased gradually from cancerous to normal mucosae, whereas p21 CIP1/WAF1 expression displayed an opposite trend. eIF4E expression decreased from cancerous to normal mucosae. Ki-67 expression, the heteroploidy ratio, S-phase fraction and proliferative index decreased gradually with the distance from the tumour centre. Based on these results, we suggest that the resection boundary in OSCC surgery should be beyond 2 cm from the tumour. Additionally, the adjacent tissues of the primary focus could be used as a model for assessing cancer progression.
Keywords: eIF4E, molecular boundary, oral squamous cell carcinoma, p21, p53
Introduction
Oral squamous cell carcinoma (OSCC), which is the most common malignant tumour in the oral and maxillofacial regions, accounts for more than 80% of all cases. 1 Although many new treatment strategies have been developed, the 5-year survival rate of patients has remained at approximately 55% for the past 30 years. One of the primary reasons for treatment failure is local recurrence. 2
Surgery, during which verifying the excision boundary is crucial, remains the most important treatment for OSCC. 3 Frozen sectioning is a pathological laboratory procedure used to perform rapid microscopic analysis of a specimen and is the most common method to determine the resection boundary of OSCC. However, the collection of sections poses a risk to patients whose adjacent tissues could become a source for local recurrence. 3,4 Although these adjacent tissues seem ‘normal', they are contiguous with tumours and could be precancerous tissues that are undergoing malignant progression. Gene expression or metabolism in tumour tissues significantly changed before any histopathological manifestations, including the overexpression of oncogenes or the malfunction of cancer-related molecules, were observed. 5
Some clinical studies have shown that the local recurrence rate of patients with histologically negative margin tissues could be 9%–32%. 6 Dolcetti et al. 7 reported that a mutated p53 gene was overexpressed in the adjacent ‘normal' or hyperplastic mucosa of laryngeal cancer and stated that p53 abnormalities most likely occur extremely early in laryngeal squamous cell carcinoma development. Curran et al. 8 showed that 35% of adjacent histologically normal samples were telomerase positive in oral cancer patients. We hypothesize that if the molecular boundary of the adjacent tissue can be defined as a surgical margin, then precancerous tissues can be fully removed to reduce the postoperative local recurrence rate effectively. Simultaneously, adjacent tissues of head and neck cancer are an ideal model to study the development and occurrence of tumours and may become a new model for head and neck cancer research.
To verify the molecular boundary of OSCC and to explore the biochemical events in OSCC progression, we conducted a study of tongue and buccal cancer biopsy samples. Histopathology, immunohistochemistry and flow cytometry were used to compare the differences among OSCCs, adjacent tissues and normal oral mucosae. Histological changes; p53, p21 CIP1/WAF1 and eIF4E expression; DNA content and sample ploidies were analysed to examine the adjacent tissues at the molecular level, which may provide a theoretical basis for the determination of a safe surgical margin.
Materials and methods
Ethics statement
This study was approved by the Institutional Review Board of West China Hospital of Stomatology, Sichuan University. All enrolled patients provided written informed consent. This study was performed according to the tenets of the Declaration of Helsinki.
Patients and samples
From 2008 to 2009, 50 patients who were treated at the Department of Head and Neck Oncology of West China Hospital of Stomatology, Sichuan University, were randomly selected to participate in the present research. Simultaneously, normal mucosa samples were donated from patients who received surgical treatment for trauma, maxillofacial malformation, or sublingual gland cysts. The clinical and pathological information for all participants is listed in Table 1. None of the patients had uncontrolled infection or immuno-deficiency disease or received any anticancer treatments for 3 months before the study. All pathological diagnoses were performed by experienced pathologists using haematoxylin and eosin (H&E) slides according to the 2005 World Health Organization histological classification. 9
Table 1. Clinical and pathological characteristics of all participants.
| Case information | Cancer (n=50) | Normal (n=20) |
|---|---|---|
| Ages/years | 31–73 (median: 54) | 33–68 (median: 50) |
| Gender | ||
| Male | 32 | 14 |
| Female | 18 | 6 |
| Sites | ||
| Tongue | 28 | 5 |
| Bucca | 22 | 15 |
| Tumor classification | ||
| T1 | 8 | NA |
| T2 | 32 | NA |
| T3 | 8 | NA |
| T4 | 2 | NA |
| Lymphatic metastasis | ||
| N0 | 4 | NA |
| N1 | 6 | NA |
| N2 | 40 | NA |
| N3 | 0 | NA |
| Distant metastasis | 0 | NA |
| Tumor grading | ||
| I | 16 | NA |
| II | 32 | NA |
| III | 2 | NA |
| Post-treatment Recurrence | 12 | NA |
| Post-treatment Metastasis | 0 | NA |
NA, not applied.
All tumour patients underwent extensive resectioning of the primary focus, combined with radical or functional neck dissection as a basic treatment. The surgical boundary was estimated during the operation, with the criterion of a minimum of 2 cm from the tumour tissues. The specimens were cut and separated into the following 5 groups: T (centre of the tumour), P1 (0–0.5 cm to the tumour boundary), P2 (0.5–1 cm to the tumour boundary), P3 (1–1.5 cm to the tumour boundary) and P4 (1.5–2 cm to the tumour boundary). Twenty normal oral mucosal specimens were selected as controls and labelled N. Patients returned for periodic post-treatment physical examination, with follow-up by phone and mail, reflecting our standard practice.
Histopathology
Biopsy samples were collected during surgery. The specimens were immediately fixed in 10% formalin, embedded in paraffin and cut into 5-μm serial sections. The sections were examined using H&E staining. The slides were observed under a light microscope to calculate the sub-mucosal invasion of malignant cells in the P1, P2, P3 and P4 groups and to identify the largest areas of sub-mucosal infiltration. Morphological changes in the adjacent epithelium were also noted.
Immunohistochemical staining
p53, p21 CIP1/WAF1 and eIF4E expression in the adjacent tissues of OSCC was evaluated by immunohistochemistry. The specimens were fixed immediately in 10% formalin, embedded in paraffin, and cut into 10-μm serial sections. After deparaffinization, the antigen unmasking procedures were performed by bringing the slices to a boil in 10 mmol·L-1 sodium citrate buffer (pH 6.0) for Ki-67 and eIF4E or in 10 mmol·L-1 elhylene diamine tetraacetic acid (EDTA) buffer (pH 8.0) for p53 and p21 CIP1/WAF 1. The slices were cooled down and rinsed with phosphate-buffered saline (PBS). Blocking buffer (5% bovine serum albumin) was added to the slides for 20 min, and then the slices were incubated with primary antibody (p53, 1:50 dilution, Abcam, Shanghai, China, product code: ab32049; p21 CIP1/WAF1 , 1:50 dilution, Cell Signaling, Danvers, MA, USA, product code: #2946; eIF4E, 1:40 dilution, Santa Cruz Biotechnology, Shanghai, China; Ki-67, 1:150 dilution, Abcam, Shanghai, China, product code: sc-9976) at 4 °C overnight. The slices were incubated in secondary antibody at 37 °C for 30 min, then incubated in horseradish peroxidase-labelled streptavidin and 3,3'-diaminobenzidine (DAB) and rinsed. Next, the slices were counterstained in haematoxylin and sealed with cover slips.
Positive p53 and p21 CIP1/WAF1 expression was defined by the presence of brown nuclear staining in the cells, whereas positive eIF4E expression was defined by the presence of brown peri-nuclear staining. The evaluation of p53, p21 CIP1/WAF1 and eIF4E staining was based on the immunoreactive score, which is a semi-quantitative method. 10,11 Grading of the samples depended on the staining intensity and positive cell percentage. Scores were determined by multiplying the intensity of the staining value (0 for negative, 1 for light, 2 for intermediate and 3 for strong staining) by the positive cell percentage (0 for 0–5%, 1 for 6%–25%, 2 for 26%–50%, 3 for 51%–75% and 4 for>75%). A score of 0 indicated negative expression; 1–4, (+); 5–8, (++) and >8, (+++). The Ki-67 proliferative index was calculated by the average percentage of positive cells in ten random fields of immunohistochemical slices at a magnification of ×400. All tissue sections were evaluated blindly by three experienced pathologists.
Flow cytometry analysis
Fresh specimens were minced with scissors, forced through an iron mesh (pore size 140 μm) and washed twice with PBS. Then, the samples were incubated in 0.25% trypsin solution at 37 °C for 20 min and fixed overnight with cold 70% ethanol. Cells were suspended at 1×106 mL−1. Propidium iodide solution (50 μg⋅mL−1) was used to stain the cells for flow cytometry. Human lymphocytes were processed in parallel with the tumour samples and used as an external diploid control. DNA analysis was performed using a flow cytometer (Coulter Elite ESP, Miami, FL, USA), and the parameters, which included the aneuploidy rate, DNA index, S-phase fraction (SPF) and proliferative index (PI) of each specimen, were recorded.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 13.0 statistics software (SPSS, Chicago, IL, USA). The Chi-square test, four-fold table Chi-square test and Fisher's exact test were performed. Kaplan–Meier curves with log-rank tests were created to assess the overall and tumour-free survival rates. Data were considered statistically significant when P>0.05.
Results
Sub-mucosa invasion extended 1 cm from the cancer boundary
Typical transitions of histopathological manifestations from OSCC to normal were observed in the H&E slides (Table 2). Twenty-one specimens had sub-mucosal OSCC invasion in the P1 group, which accounted for 42% of all cases, whereas only 8 specimens had OSCC invasion in the P2 group, which accounted for 16% of all cases. No sub-mucosal invasion was found in the P3 and P4 groups (Figure 1a). Significant differences in the sub-mucosal invasion rates were observed among the P1, P2 and P3, P4 groups (P<0.001), and no significant difference in the sub-mucosal invasion rates was found between the P3 and P4 groups (P=1.000).
Table 2. H&E staining of para-tumor tissue.
| Groups | Case number | Normal or simple hyperplasia | Mild or moderate dysplasia | Severe dysplasia | Submucosal invasion of cancer | Invasion rate/% |
|---|---|---|---|---|---|---|
| P1 | 50 | 0 | 27 | 23 | 21 | 42a |
| P2 | 50 | 11 | 31 | 8 | 8 | 16a |
| P3 | 50 | 32 | 18 | 0 | 0 | 0a , b |
| P4 | 50 | 47 | 3 | 0 | 0 | 0a , b |
H&E, haematoxylin and eosin. P1, 0–0.5 cm to tumor boundary; P2, 0.5–1 cm to tumor boundary; P3, 1–1.5 cm to tumor boundary; P4, 1.5–2 cm to tumor boundary.
Significant differences of sub-mucosal invasion rate were observed between P1, P2 and P3, P4 groups (P<0.001).
No statistical difference of sub-mucosal invasion rate was found between P3 and P4 groups (P=1.000).
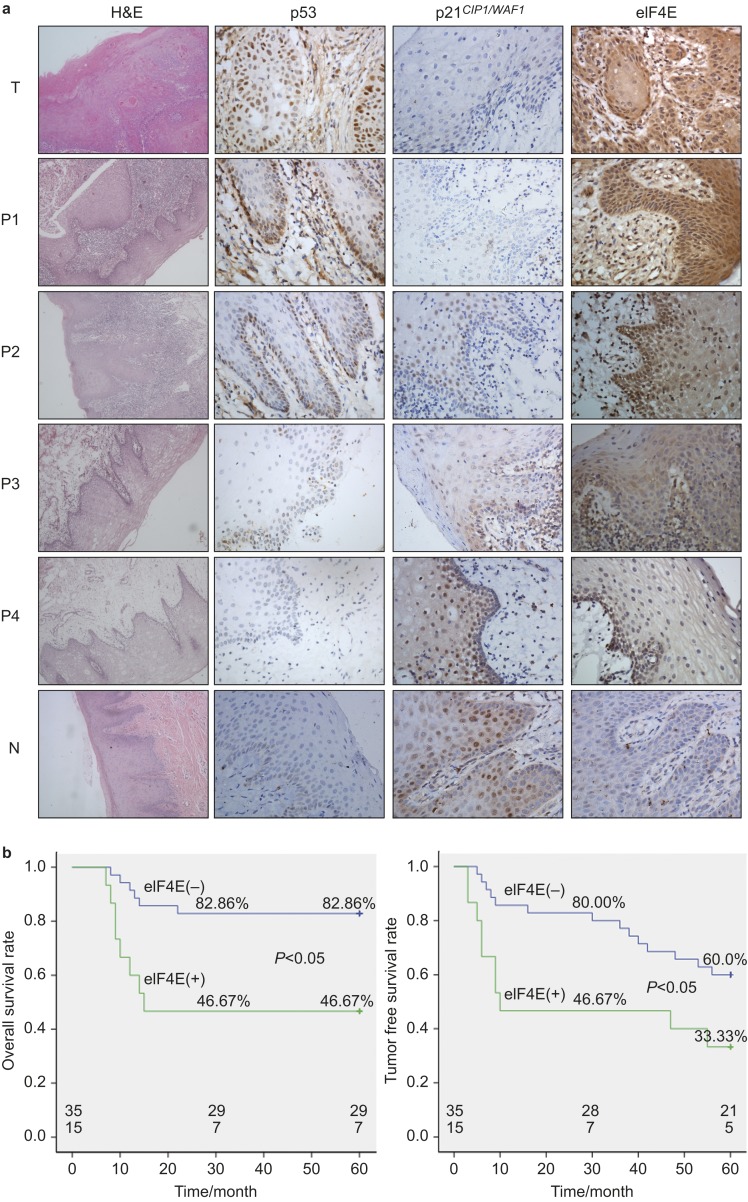
Figure 1.
Sub-mucosal invasion and p53, p21 CIP1/WAF and eIF4E expression in the tumour and adjacent tissues. (a) Biopsies of primary lesions and the adjacent tissues from patients were taken and divided into the following groups: T, centre of the tumour; P1, 0–0.5 cm to the tumour boundary; P2, 0.5–1 cm to the tumour boundary; P3, 1–1.5 cm to the tumour boundary; P4, 1.5–2 cm to the tumour boundary; N, normal mucosa. Then, the samples processed for immunoreactivity to p53, p21 CIP1/WAF and eIF4E. H&E staining is presented at ×100 magnification, whereas all other panels of immunohistochemical staining are presented at ×400 magnification. (b) Overall and tumour-free survival rates for the patients based on the immunohistochemical staining of eIF4E in the P4 (1.5–2 cm to the tumour boundary) regions. H&E, haematoxylin and eosin.
Changes in p53 and p21 CIP1/WAF1 expression were observed in the para-cancer mucosa
Brown staining indicative of p53 expression was observed in the nuclei of tumour cells in the immunohistochemical slides. The P1, P2, P3 and P4 groups showed brown nuclear staining in the basal and prickle cell layers, whereas no staining was observed in normal oral mucosa (Figure 1a). Positive p53 expression decreased gradually from P1 group to P4 group. The positive staining ratios were 68% (34/50) in the tumour samples (T), 60% (30/50) in P1 group, 46% (23/50) in P2 group and 18% (9/50) in P3 group. However, 0 of P4 group samples displayed positive staining (0/50) (Table 3). Significant differences in p53 expression were observed between the T, P1, P2 and P3, P4 groups (P<0.05). No significant difference in p53 expression was found between T and the P1 and P2 groups (P>0.05) and between P4 and N groups (P>0.05).
Table 3. Immunohistochemical gradient of P53 and P21 CIP1/ WAF1 in OSCC, para-tumor tissue and normal mucosa.
| Gradation of staining | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Number | - | + | ++ | +++ | Positive | Positive rate/% |
| P53 | |||||||
| T | 50 | 16 | 5 | 16 | 13 | 34 | 68a |
| P1 | 50 | 20 | 10 | 12 | 8 | 30 | 60a |
| P2 | 50 | 27 | 12 | 7 | 4 | 23 | 46a |
| P3 | 50 | 41 | 5 | 4 | 0 | 9 | 18a |
| P4 | 50 | 50 | 0 | 0 | 0 | 0 | 0a |
| N | 20 | 20 | 0 | 0 | 0 | 0 | 0a |
| P21 CIP1/ WAF1 | |||||||
| T | 50 | 32 | 13 | 5 | 0 | 18 | 36b |
| P1 | 50 | 30 | 15 | 5 | 0 | 20 | 40b |
| P2 | 50 | 26 | 17 | 7 | 0 | 24 | 48b |
| P3 | 50 | 17 | 15 | 18 | 0 | 33 | 66b |
| P4 | 50 | 2 | 16 | 30 | 2 | 48 | 96b |
| N | 20 | 0 | 8 | 10 | 2 | 20 | 100b |
| eIF4E | |||||||
| T | 50 | 0 | 0 | 29 | 21 | 50 | 100c |
| P1 | 50 | 14 | 5 | 18 | 13 | 36 | 72c |
| P2 | 50 | 26 | 8 | 12 | 4 | 24 | 48c |
| P3 | 50 | 31 | 11 | 8 | 0 | 19 | 38c |
| P4 | 50 | 35 | 12 | 3 | 0 | 15 | 30c |
| N | 20 | 20 | 0 | 0 | 0 | 0 | 0c |
OSCC, oral squamous cell carcinoma. T, centre of the tumor; P1, 0–0.5 cm to tumor boundary; P2, 0.5–1 cm to tumor boundary; P3, 1–1.5 cm to tumor boundary; P4, 1.5–2 cm to tumor boundary; N, normal mucosa.
Significant differences of p53 expression were observed between T, P1, P2 and P3, P4 groups (P<0.05). No statistical difference of p53 expression was found between T and P1, P2 groups (P>0.05) and between P4 and N groups (P>0.05).
No significant differences between T, P1 and P2 groups (P>0.05), or between P4 and N groups (P>0.05); statistical differences between P2 and P3 groups (P<0.001), and between P3 and P4 groups (P<0.001).
Significant differences of eIF4E expression were observed between T, P1, P2, P3, P4 and N groups (P<0.005).
Positive p21 CIP1/WAF1 staining was localized primarily to the cell nucleus. Negligible p21 CIP1/WAF1 signal was observed in tumour samples (T) and in tissue samples 0–0.5 cm to the tumour boundary (P1). As the distance from the tumour foci increased, greater numbers of positive cells were detected in the basal and suprabasal strata in the adjacent tissues (Figure 1a). The positive staining ratios were 36% (18/50) in the tumour samples (T), 40% (20/50) in P1 group, 48% (24/50) in P2 group, 66% (33/50) in P3 group, 96% (48/50) in P4 group and 100% (20/20) in the normal samples (Table 3). No significant differences were observed between T, P1 and P2 groups (P>0.05) or between P4 and N groups (P>0.05). However, significant differences in p21 CIP1/WAF1 expression could be found between P2 and P3 groups (P<0.001) and between P3 and P4 groups (P<0.001). Therefore, the cells of the P1 and P2 groups were similar to the cancer cells, whereas the cells of the P4 group might not yet have undergone the pre-malignant process. The cells in the P3 group could be transitional subpopulations that are in the process of malignant transformation.
eIF4E expression decreased from cancerous to normal tissues
Cells positive for eIF4E expression were characterized by brown perinuclear and cytoplasmic brown staining (Figure 1a). In the tumour centre, positive cells were diffused throughout the entire epithelia. In the adjacent mucosa, as the distance from the tumour foci increased, the staining intensity significantly decreased, and the positive cells were found primarily in the basal and suprabasal strata. The positive staining ratios were 100% (50/50) in the tumour samples (T), 72% (36/50) in P1 group, 48% (24/50) in P2 group, 38% (19/50) in P3 group and 30% (15/50) in P4 group. eIF4E-positive cells were not observed in the normal oral mucosa specimens. eIF4E expression decreased from the centre to outer regions but still displayed 30% positive expression in P4 group, which significantly differed from that of the N group (P<0.005) (Table 3).
During the follow-up period, 6 patients (6/35, 17.14%) with negative eIF4E staining and 8 (8/15, 53.33%) patients with positive eIF4E staining in the P4 group died. Additionally, 14 (14/35, 40%) patients with negative eIF4E staining and 10 (10/15, 66.67%) patients with positive eIF4E staining had post-treatment recurrences (Figure 1b). Survival was evaluated for the entire population. A log-rank test for the overall survival rate that compared the two groups indicated that patients with negative eIF4E staining in the mucosa 1.5–2 cm from the tumour had a significantly higher survival rate than those patients with positive eIF4E staining (P=0.004). Similar results were observed for the tumour-free survival rate when comparing the two groups (P=0.031). These results suggested us that positive eIF4E staining in the adjacent tissues of OSCC could be used as a parameter to predict clinical outcomes.
Proliferative assay of the cells in cancerous and adjacent mucosa
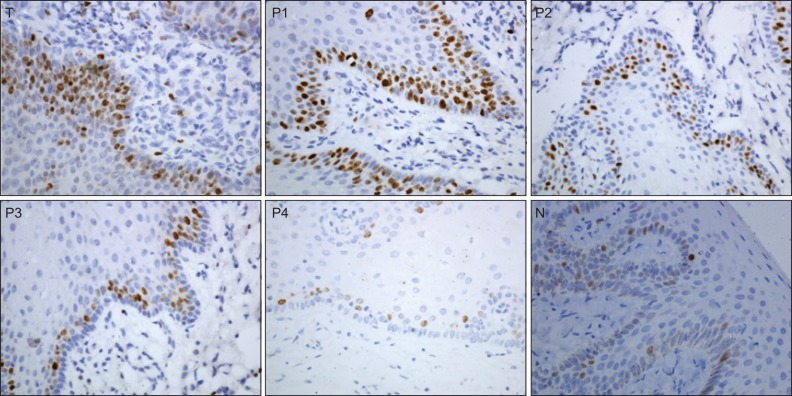
In the present study, we used Ki-67 expression as an index to determine the proliferative ability of the cells in the cancerous and adjacent mucosa. We observed that positive Ki-67 staining was characterized by nuclear brown staining (Figure 2). In the tumour centre, positive cells were gathered in the tumour focus or were arranged in a ball or cord within the islets and were distributed in the basal or spinous layer of the adjacent mucosa. A gradual decrease in the Ki-67 proliferative index was observed from the tumour focus (31.07%±4.39%) and the P1 group (40.10%±6.44%) to the P4 (7.11%±2.76%) and normal groups (6.74%±3.32%). Using chi-square analysis, significant differences were observed among the groups (F=480.535, P<0.001), while no difference was found between the P4 and N groups (P>0.05) (Table 4).
Figure 2.
Immunohistochemical staining of Ki-67 in the tumour and adjacent tissues (×400). T, centre of the tumour; P1, 0–0.5 cm to the tumour boundary; P2, 0.5–1 cm to the tumour boundary; P3, 1–1.5 cm to the tumour boundary; P4, 1.5–2 cm to the tumour boundary; N, normal mucosa.
Table 4. Proliferative index of Ki-67 and flow cytometry analysis in OSCC, para-tumor tissue and normal mucosa.
| Groups | Number | PI of Ki-67 #/% | Aneuploidy | Aneuploidy rate/% | SPF #/% | PI #/% |
|---|---|---|---|---|---|---|
| T | 50 | 31.07±4.39a | 31 | 62b | 27.51±6.69c | 30.27±6.59d |
| P1 | 50 | 40.10±6.44a | 27 | 54b | 25.96±6.56c | 28.55±6.44d |
| P2 | 50 | 18.61±1.91a | 14 | 28b | 21.50±5.72c | 23.72±5.29d |
| P3 | 50 | 13.96±3.91a | 8 | 16b | 17.60±5.79c | 19.63±5.80d |
| P4 | 50 | 7.11±2.76a , e | 8 | 16b | 14.26±5.84c | 16.12±5.69d |
| N | 20 | 6.74±3.32a , e | 0 | 0b | 8.39±1.93c | 10.85±2.04d |
OSCC, oral squamous cell carcinoma; PI, proliferative index; SPF, S-phase fraction. T, centre of the tumor; P1, 0–0.5 cm to tumor boundary; P2, 0.5–1 cm to tumor boundary; P3, 1–1.5 cm to tumor boundary; P4, 1.5–2 cm to tumor boundary; N, normal mucosa.
#Mean±Standard deviation.
Significant statistic differences among the groups (P<0.001).
Significant differences between T, P1 and P2, P3, P4, N groups (P<0.001), and between N with T, P1, P2, P3 and P4 groups (P<0.001), no difference was found between T and P1 groups (P>0.05).
There were no statistical difference between P4 and N groups (P>0.05).
Differences in both DNA content and ploidy were found among the samples. Ratios of aneuploidy, SPF and PI gradually with increasing distance from the tumour centre. When the chi-square test was used, significant differences in the ratio of aneuploidy were found between T and the P2, P3, P4 and N groups (P<0.001); between P1 and the P2, P3, P4 and N groups (P<0.001); and between N and the T, P1, P2, P3 and P4 groups (P<0.001). In contrast, no difference was found between T and P1 groups (P>0.05). SPF and PI analyses showed similar results regarding the ratio of aneuploidy among the cancer tissues (Table 4).
Discussion
In this study, H&E staining illustrated a typical transition of tissue morphology from OSCC to normal. All specimens in the P1 group had typical hyperplasia, while only three specimens showed mild hyperplasia in the P4 group. Twenty-one (42%) specimens in the P1 group had sub-mucosal OSCC invasion, while only three specimens (16%) in the P2 group had OSCC invasion. No sub-mucosal invasion was found in the region 1 cm away from tumour tissues. Other investigators have reported that the sub-mucosal invasion border of buccal squamous cell carcinoma can be up to a width of 9.99 mm, 12,13 which is consistent with our study. At the region 1.0–1.5 cm from the visible borders of the tumour, 18 cases with moderate to mild epithelial dysplasia were observed. Mild dysplasias were found in three cases at the region 1.5–2.0 cm to the tumour boundary, and the rest were simple hyperplasia or normal. Thus, according to our histopathological observations of adjacent OSCC tissues, combined with existing research findings, 1.5 cm away from visible tumour tissues can be considered a histological boundary for clinical treatments.
Previous studies have shown that abnormalities occur at the molecular level of tumour cells before histomorphological changes. Ngoi et al. 14 found that oncogene and tumour suppressor gene expression significantly changed in the mucosa adjacent to cancer and differed from normal mucosa; para-tumour mucosa was in a state of pre-malignancy. Therefore, exploring the molecular boundaries of OSCC para-tumour tissue has a great significance for determining the excision boundary for surgery.
The wt-p53 gene is a primary mediator of cell cycle arrest, DNA repair, and apoptosis. The mutation and inactivation of p53 may be a critical event in the origin and progression of head and neck carcinoma, and p53 mutations are among the most common genetic changes in oral squamous cell carcinoma. 7,15 In recent years, the p53 overexpression rate for oral squamous cell carcinoma was found to be 50%–80%, and p53 expression significantly correlated with the occurrence and metastasis of head and neck cancer and with survival time. 16,17,18,19 Other scholars also found that p53 overexpression in surgical margin tissue is one of the indicators of a poor prognosis and recurrence and that p53 expression in the surgical margin can provide the basis for filtering high-risk cases of tumour recurrence. 17,20 Our study found that positive p53 expression decreased gradually from group P1 to P4. No significant difference in p53 expression was found between T and the P1 and P2 groups (P>0.05) or between normal oral mucosa and regions 1.5 cm and farther from the centre of the tumour (P>0.05). However, significant differences between the regions from the tumour to 1.0 cm from the visible boundary and the regions from 1.5 cm away from the tumour and normal mucosa were found. Therefore, we believe that the molecular boundary of p53 is 1.5 cm away from the visible boundary of the tumour tissues.
p21 is a potent cyclin-dependent kinase inhibitor. As an inhibitor of cyclin E/cdk2 and cyclin A/cdk2 and, to a lesser extent, of cyclin B/cdk1, p21 CIP1/WAF1 functions as a regulator of cell cycle progression at G1 and is necessary for cell cycle entry and progression. 21,22 The tumour suppressor protein p53 can precisely control the expression of the p21 gene, which mediates the p53-dependent cell cycle G1 phase arrest in response to a variety of stress stimuli. However, in post-mitotic differentiated cells, such as spinous cells in squamous epithelia, p21 CIP1/WAF1 expression is differentiation-dependent rather than p53-dependent. Despite this dependence, loss-of-function p21 mutations do not accumulate in cancer nor do they predispose individuals to cancer incidence. 23,24 Similar to these results, we found that positive p21 CIP1/WAF1 expression was rare in the tumour proper. As the distance increased, greater numbers of positive cells were detected in the basal and suprabasal strata. Additionally, the results showed that the samples in the P1 and P2 groups (1.0 cm to the visible tumour boundary) were similar to cancer samples, whereas P4 group (2.0 cm to the tumour boundary) was similar to normal mucosa. The P3 group (1.0–1.5 cm to the tumour) appeared to be a transitional area that differed from the cancerous and normal mucosa. Therefore, we believe that the molecular boundary of p21 is 1.5 cm away from the visible boundary of the tumour tissues.
eIF4E, which is a eukaryotic protein synthesis initiation factor, is elevated in all head and neck squamous cell carcinomas. 25,26,27 Sorrells et al. 28 confirmed that the eIF4E gene was amplified and overexpressed in head and neck squamous cell carcinoma. eIF4E amplification and elevation were the highest in the tumour core, intermediate in the transitional zone, and lowest in the tumour-free margin. eIF4E gene amplification and overexpression appeared to progress from the ‘tumour-free' margin to the tumour core. In our study, eIF4E decreased from the centre to outer regions but still had 30% positive expression at regions 2.0 cm from the visible borders of the tumour, showing a significant difference compared with normal oral mucosa (P<0.005). We believe that the boundary of eIF4E is at least 2 cm away from the tumour. At present, however, without efficient methods for reconstruction of oral organs, the surgical margins of OSCC are generally less than 2 cm away from the visible borders of lesions due to the consideration of the post-operative function of organs and special local anatomical structure. Thus, we could not obtain biopsy samples more than 2 cm from the visible tumour boundary to define the eIF4E molecular border more accurately.
Significantly, we found that eIF4E overexpression may be an earlier molecular event than p53 mutation in the occurrence of OSCC. Franklin et al. 29 showed that eIF4E expression in surgical margins was an independent prognostic factor and that its absence in surgical margins might predict long-term survival; detecting eIF4E expression in the surgical margins might improve survival by determining which patients would benefit from further resection or adjuvant therapy. Nathan et al. 30 demonstrated that eIF4E is a more sensitive and effective recurrent predictor of head and neck squamous cell carcinoma compared with p53. Our retrospective research regarding clinical outcomes showed similar results; patients with positive eIF4E staining in P4 regions (2.0 cm to the tumour boundary) suffered higher recurrence and mortality rates (P<0.05). Therefore, we believe that eIF4E is a more precise determinant of the molecular border of OSCC, and we suggest that head and neck oncologists could use immunohistochemical staining of eIF4E in the adjacent tissues of OSCC as a parameter for adjunctive treatments after primary surgery.
Ki-67 expression has been used as a well-developed assay in many studies to analyse cancer cell proliferation. 31,32 In the present research, we found a gradual decrease in Ki-67 expression from the tumour and P1 group to the P4 group and normal mucosa, which indicated that the proliferative ability decreased from cancerous to normal mucosae. Interestingly, the proliferative index of Ki-67 in the P1 group (40.10±6.44) was higher than that in tumour samples (31.07±4.39). We supposed that the cells in the P1 group (0–0.5 cm to the tumour boundary) might have the most active proliferative and invasive abilities; this region is called the invasive tumour front. 33 However, these results require further studies. Additionally, based on Ki-67 expression, we determined that the safe surgical boundary should be at least 1.5 cm from the visible tumour margin. Many studies have confirmed that when malignant tumours occur, abnormal DNA content and cell proliferative activity are important biological characteristics of the malignant cells. 34,35 The DNA content of diploid cells is strictly consistent in normal tissues, while the DNA content of malignant cells is often higher. DNA aneuploid cells arise in 70%–90% of malignant tumours. 33,35 DNA ploidy and the SPF are important indicators of abnormal tumour cell proliferation. Therefore, quantitative research regarding DNA content and ploidy can objectively reflect the aggressive biological behaviour of malignant cells, such as their proliferative and invasive activities. 36 We found that the aneuploidy rate, SPF and PI gradually decreased with the increasing distance of samples from the tumour centre. Significant differences still existed at the region 2 cm away from tumour tissues, which indicated that the boundaries of these indexes were larger than 2 cm.
Human papillomavirus (HPV) is now recognized as one of the primary causes of head and neck cancers. 37 HPV infects the epithelium of skin and mucous membranes selectively. In total, 150 HPV genotypes have been identified and fully sequenced; of theses genotypes, the most common types in oral carcinomas are HPV 16, 33 and 82. 37,38 Most (>90%) HPV-associated head and neck squamous cell cancers are caused by HPV 16. The molecular mechanism of HPV carcinogenesis can be explained by the regulation and function of the two viral oncogenes E6 and E7. The E6 gene product binds to the p53 tumour suppressor gene. The association of E6 with p53 leads to the specific ubiquitination and degradation of the p53 protein. This degradation then prevents p53 from inducing either the growth arrest or apoptosis of infected cells1. 39,40 Gillison et al. 41 reported that HPV-16 DNA was detected in head and neck cancers of 92 (38%) of 240 case subjects by in situ hybridization in their clinical research. Because the E6 protein of HPV16 destabilizes p53, HPV16-positive carcinomas are likely to be negative for the stable, mutated p53 protein detected in the present study, which might be the reason that 32% of the samples in the T group displayed negative p53 staining. We did not detect HPV in the present study. A method of HPV detection that is highly accurate, reproducible from one diagnostic laboratory to the next, and practical for universal application in the clinical arena, is a pressing need. We suggest that HPV detection should be one of the routine exams used for the head and neck cancer patients in the future.
According to our histopathological and molecular pathological studies of OSCC para-tumour tissues, we reached the following conclusions: the gradient expression of p53 and eIF4E in para-tumour tissues indicates that molecular boundaries exist within the para-tumour tissue. eIF4E overexpression may be an earlier molecular event than p53 mutations during OSCC progression. Therefore, using eIF4E expression to define the molecular boundary of para-tumour tissue, which is 2 cm away from the visible borders of tumours, may be more appropriate. Para-tumour tissues have a high potential for malignant transformation and should be resected thoroughly to reduce the recurrence of OSCC. If possible, a 2-cm clinical boundary should be secured during operation. Otherwise, a minimum 1.5-cm boundary should be secured. Additionally, post-operational chemo- and radiotherapies should be provided to reinforce the elimination of malignant tissues and cells.
Acknowledgments
The project was supported by the National Natural Science Foundation of China (Grant Nos. 81001209 and 81172578) and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (Grant No. 81321002). We would like to thank Dr. James J. Cody from the University of Alabama at Birmingham for his kind help in the manuscript preparation.
References
- 1McDowell JD. An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol Clin North Am 2006; 39(2): 277–294. [DOI] [PubMed] [Google Scholar]
- 2Yanamoto S, Yamada S, Takahashi Het al. Clinicopathological risk factors for local recurrence in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2012; 41(10): 1195–1200. [DOI] [PubMed] [Google Scholar]
- 3Guillemaud JP, Patel RS, Goldstein DPet al. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol Head Neck Surg 2010; 39(4): 370–377. [PubMed] [Google Scholar]
- 4Brandwein-Gensler M, Teixeira MS, Lewis CMet al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 2005; 29(2): 167–178. [DOI] [PubMed] [Google Scholar]
- 5Lyons AJ, Jones J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int J Oral Maxillofac Surg 2007; 36(8): 671–679. [DOI] [PubMed] [Google Scholar]
- 6Jacobs JR, Ahmad K, Casiano Ret al. Implications of positive surgical margins. Laryngoscope 1993; 103(1 Pt 1): 64–68. [DOI] [PubMed] [Google Scholar]
- 7Dolcetti R, Doglioni C, Maestro Ret al. p53 over-expression is an early event in the development of human squamous-cell carcinoma of the larynx: genetic and prognostic implications. Int J Cancer 1992; 52(2): 178–182. [DOI] [PubMed] [Google Scholar]
- 8Curran AJ, St Denis K, Irish Jet al. Telomerase activity in oral squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 1998; 124(7): 784–788. [DOI] [PubMed] [Google Scholar]
- 9Gale N, Plich BZ, Sidransky Det al. Tumours of the hypopharynx, larynx and trachea (Epithelial precursor lesions)//Barnes L, Eveson JW, Reichart P et al. eds. World Health Organization Classification of Tumours Pathology & Genetics Head and Neck Tumours International Agency for Research on Cancer (IARC). Lyon: IARC Press, 2005: 140–143. [Google Scholar]
- 10Stark AM, Hugo HH, Tscheslog Het al. p53, BCL-2 and BAX in non-small cell lung cancer brain metastases: a comparison of real-time RT-PCR, ELISA and immunohistochemical techniques. Neurol Res 2007; 29(5): 435–440. [DOI] [PubMed] [Google Scholar]
- 11Schindlbeck C, Hantschmann P, Zerzer Met al. Prognostic impact of KI67, p53, human epithelial growth factor receptor 2, topoisomerase IIalpha, epidermal growth factor receptor, and nm23 expression of ovarian carcinomas and disseminated tumor cells in the bone marrow. Int J Gynecol Cancer 2007; 17(5): 1047–1055. [DOI] [PubMed] [Google Scholar]
- 12Chiou WY, Lin HY, Hsu FCet al. Buccal mucosa carcinoma: surgical margin less than 3 mm, not 5 mm, predicts locoregional recurrence. Radiat Oncol 2010; 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Ota Y, Aoki T, Karakida Ket al. Determination of deep surgical margin based on anatomical architecture for local control of squamous cell carcinoma of the buccal mucosa. Oral Oncol 2009; 45(7): 605–609. [DOI] [PubMed] [Google Scholar]
- 14Ngoi SS, Staiano-Coico L, Godwin TAet al. Abnormal DNA ploidy and proliferative patterns in superficial colonic epithelium adjacent to colorectal cancer. Cancer 1990; 66(5): 953–959. [DOI] [PubMed] [Google Scholar]
- 15Hoffmann TK, Bier H, Donnenberg ADet al. p53 as an immunotherapeutic target in head and neck cancer. Adv Otorhinolaryngol 2005; 62: 151–160. [DOI] [PubMed] [Google Scholar]
- 16Carlos de Vicente J, Junquera Gutiérrez LM, Zapatero AHet al. Prognostic significance of p53 expression in oral squamous cell carcinoma without neck node metastases. Head Neck 2004; 26(1): 22–30. [DOI] [PubMed] [Google Scholar]
- 17Heah KG, Hassan MI, Huat SC. p53 expression as a marker of microinvasion in oral squamous cell carcinoma. Asian Pac J Cancer Prev 2011; 12(4): 1017–1022. [PubMed] [Google Scholar]
- 18Kato K, Kawashiri S, Yoshizawa Ket al. Expression form of p53 and PCNA at the invasive front in oral squamous cell carcinoma: correlation with clinicopathological features and prognosis. J Oral Pathol Med 2011; 40(9): 693–698. [DOI] [PubMed] [Google Scholar]
- 19Perisanidis C, Perisanidis B, Wrba Fet al. Evaluation of immunohistochemical expression of p53, p21, p27, cyclin D1, and Ki67 in oral and oropharyngeal squamous cell carcinoma. J Oral Pathol Med 2012; 41(1): 40–46. [DOI] [PubMed] [Google Scholar]
- 20Allegra E, Puzzo L, Cutrona Det al. p53 overexpression on the resection margins as a marker of local recurrence in glottic T1a carcinoma. Acta Otorhinolaryngol Ital 2003; 23(6): 454–458. [PubMed] [Google Scholar]
- 21el-Deiry WS, Tokino T, Velculescu VEet al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75(4): 817–825. [DOI] [PubMed] [Google Scholar]
- 22Harper JW, Adami GR, Wei Net al. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75(4): 805–816. [DOI] [PubMed] [Google Scholar]
- 23Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 2005; 65(10): 3980–3985. [DOI] [PubMed] [Google Scholar]
- 24Bedelbaeva K, Snyder A, Gourevitch Det al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A 2010; 107(13): 5845–5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004; 23(18): 3189–3199. [DOI] [PubMed] [Google Scholar]
- 26Nathan CO, Franklin S, Abreo FWet al. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol 1999; 17(9): 2909–2914. [DOI] [PubMed] [Google Scholar]
- 27Sunavala-Dossabhoy G, Palaniyandi S, Clark Cet al. Analysis of eIF4E and 4EBP1 mRNAs in head and neck cancer. Laryngoscope 2011; 121(10): 2136–2141. [DOI] [PubMed] [Google Scholar]
- 28Sorrells DLJr, Ghali GE, de Benedetti Aet al. Progressive amplification and overexpression of the eukaryotic initiation factor 4E gene in different zones of head and neck cancers. J Oral Maxillofac Surg 1999; 57(3): 294–299. [DOI] [PubMed] [Google Scholar]
- 29Franklin S, Pho T, Abreo FWet al. Detection of the proto-oncogene eIF4E in larynx and hypopharynx cancers. Arch Otolaryngol Head Neck Surg 1999; 125(2): 177–182. [DOI] [PubMed] [Google Scholar]
- 30Nathan CO, Amirghahri N, Rice Cet al. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope 2002; 112(12): 2129–2140. [DOI] [PubMed] [Google Scholar]
- 31Babichenko II, Rabinovich OF, Ivina AAet al. [Papillomavirus in the genesis of oral leukoplakia.] Arkh Patol 2014; 76(1): 32–36. Russian. [PubMed] [Google Scholar]
- 32Myoung H, Kim MJ, Lee JHet al. Correlation of proliferative markers (Ki-67 and PCNA) with survival and lymph node metastasis in oral squamous cell carcinoma: a clinical and histopathological analysis of 113 patients. Int J Oral Maxillofac Surg 2006; 35(11): 1005–1010. [DOI] [PubMed] [Google Scholar]
- 33Akino T, Hida K, Hida Yet al. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am J Pathol 2009; 175(6): 2657–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Romics I, Bocsi J, Bach Det al. DNA content of prostatic cancer measured by flow cytometry in patients undergoing radical prostatectomy. Anticancer Res 1995; 15(3): 1131–1134. [PubMed] [Google Scholar]
- 35Bignold LP. Mutation, replicative infidelity of DNA and aneuploidy sequentially in the formation of malignant pleomorphic tumors. Histol Histopathol 2007; 22(3): 321–326. [DOI] [PubMed] [Google Scholar]
- 36Abou-Elhamd KE, Habib TN. The flow cytometric analysis of premalignant and malignant lesions in head and neck squamous cell carcinoma. Oral Oncol 2007; 43(4): 366–372. [DOI] [PubMed] [Google Scholar]
- 37Marur S, D'Souza G, Westra WHet al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11(8): 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Kero K, Rautava J, Syrjänen Ket al. Oral mucosa as a reservoir of human papillomavirus: point prevalence, genotype distribution, and incident infections among males in a 7-year prospective study. Eur Urol 2012; 62(6): 1063–1070. [DOI] [PubMed] [Google Scholar]
- 39Ruttkay-Nedecky B, Jimenez Jimenez AM, Nejdl Let al. Relevance of infection with human papillomavirus: the role of the p53 tumor suppressor protein and E6/E7 zinc finger proteins (Review). Int J Oncol 2013; 43(6): 1754–1762. [DOI] [PubMed] [Google Scholar]
- 40Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J 1991; 10(13): 4129–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Gillison ML, D'Souza G, Westra Wet al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100(6): 407–420. [DOI] [PubMed] [Google Scholar]