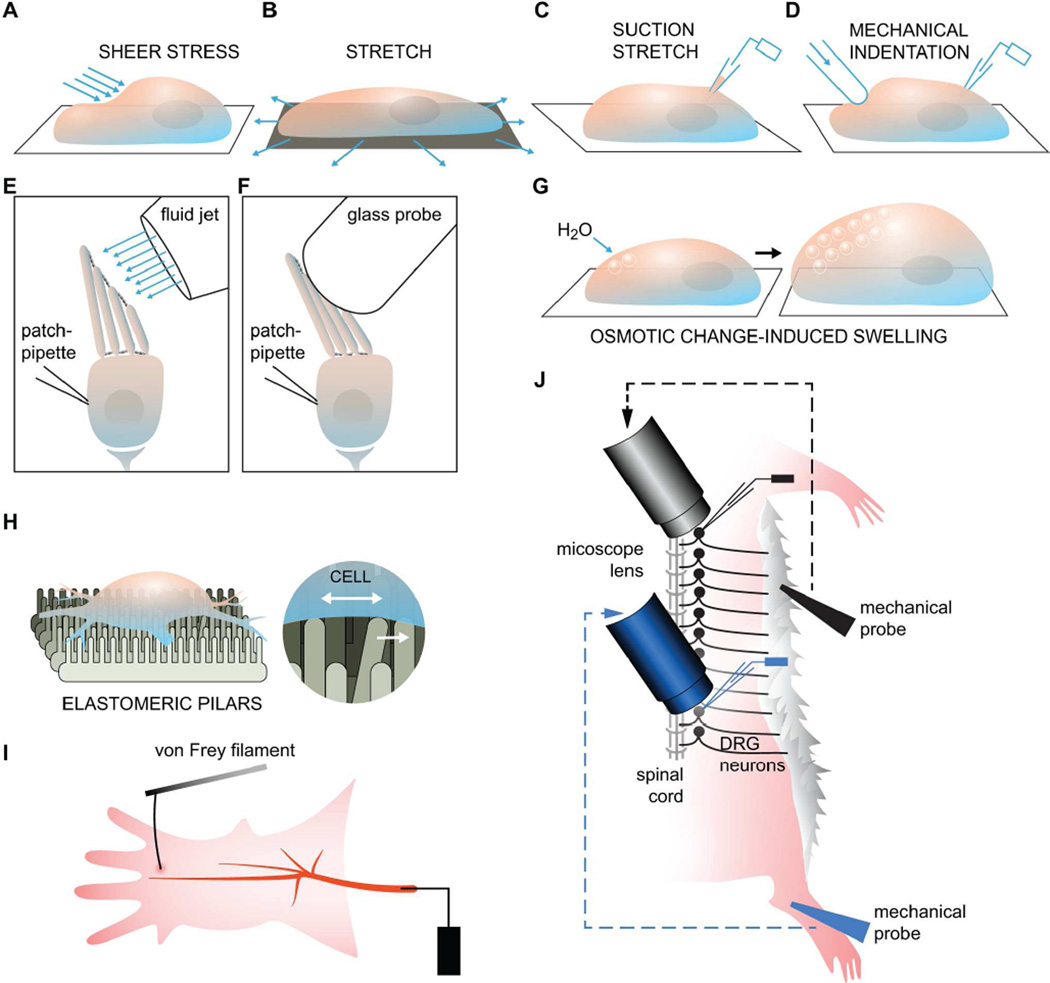
Figure 2. Mechanotransduction Assays.
(A) To generate shear stress, a perfusion tube with a small opening at the tip is placed near the cell and bath solution is then injected onto the cell membrane (McCarter et al., 1999; Olesen et al., 1988). (B and C) Membrane stretch can either be applied generally to the entire basal side of a cell or a liposome that has been seeded onto a flexible material, or, more locally, to a small patch of membrane using a glass pipette to generate a protrusion or “bleb” (Bhattacharya et al., 2008; Maingret et al., 1999a; Sukharev and Sachs, 2012). (D) Physical indentation, or poking of the cell membrane or a lipid bilayer by a glass pipette controlled by a piezoelectric device, can also be used to apply force onto a local region of the membrane (McCarter et al., 1999). (E and F) Isolated epithelial hair cells can be stimulated with either a glass probe or fluid shear stress. Both methods serve to bend the stereocilia but only the shear stress method can be used for applying force in both positive and negative directions. (G) Changes to the osmotic potential can cause an influx of water and lead to cell swelling. (H) New assays such as the elastomeric pillars have been developed to more precisely deliver mechanical strain and can be precise enough to apply force to specific parts of a cell, such as the nerve endings. (I) The classic setup for the saphenous nerve preparation used to record action potential firing upon stimulation with a von Frey filament. (J) A variant form of the skin-nerve preparation that can be used to monitor the activity of DRG neurons along the entire spinal cord.