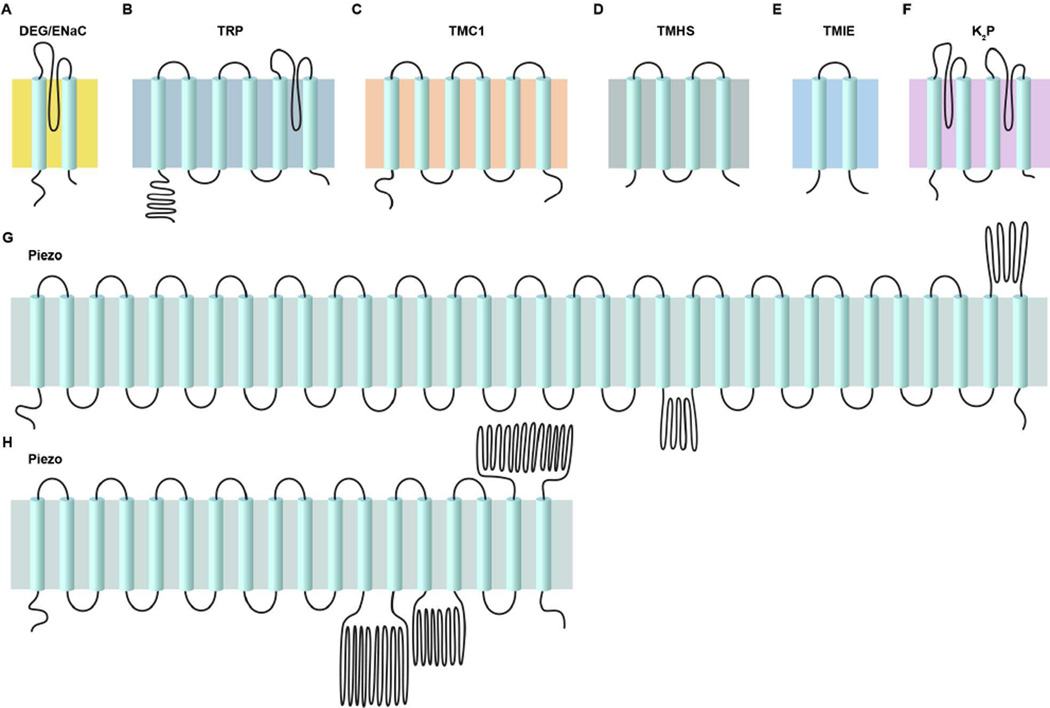
Figure 3. Eukaryotic Ion Channel Subunits.
The topology of ion channel subunits implicated in eukaryotic mechanotransduction illustrates the vast diversity compared to other sensory receptors. (A) DEG/ENaC proteins have 2 TM domains and associate as trimeric complexes. (B) The general structure of many TRP channels is a tetrameric complex where each subunit contains 4 TM domains and a pore loop domain between TM5 and TM6. An extraordinary amount of structural diversity exists between TRP channel members, particularly within the structure of the N and C terminal soluble domains of the proteins. (C – E) At present, four proteins (TMC1/2, TMIE and TMHS) have been shown to be necessary for mechanotransducer currents in hair cells. The exact composition of the various subunits to the channel complex and whether this composition varies along the tonotopic axis is unknown. (F) K2P channels are unique among other ion channel complexes in that each subunit contains 2 poor loop helices and the formation of a functional pore requires an association of dimers. (G and H) Biochemical and computational evidence suggest that Piezo ion channels contain at least 18 TM domains and potentially up to 38 TM domains.