Fig. 3.
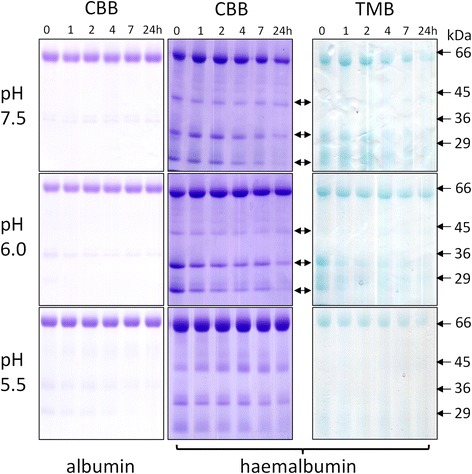
Albumin and haemalbumin breakdown by InpA at different pH under non-reducing conditions. Albumin and haemalbumin (1:1 haem:protein ratio) (16 μM) were incubated with InpA (100 μg ml−1) at 37 °C and aliquots of the incubations were sampled periodically at 1, 2, 4, 7 and 24 h and InpA activity inhibited by E-64 (0.5 mM) and subjected to SDS-PAGE on 10 % non-reducing gels. Samples were solubilised at 37 °C for 1 h in non-reducing application buffer. The haemalbumin gels were stained with TMB to reveal haem-associated peroxidise activity and counterstained with CBB for protein, whilst albumin gels were stained only with CBB. Protein loading was ≈ 20 μg per lane. Arrows denote digestion fragments carrying bound haem
