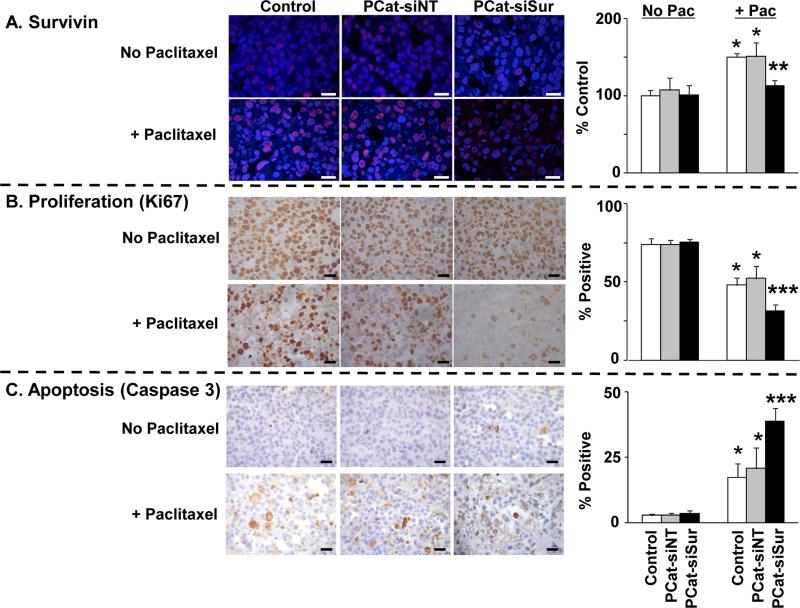
Figure 4. Paclitaxel enhanced the delivery and transfection of PCat-siSurvivin in tumors:Cellular and molecular pharmacodynamic endpoints.
Immunodeficient mice were implanted with tumors and treated as described in Figure 3. Tumors were excised 72 h after the second dose of paclitaxel treatment (96 h after siRNA treatment), and processed for immunohistochemical staining and quantitative image analysis of survivin levels and of Ki67- and caspase 3-positive cells as described in Methods. Mean±SD (3-5 microscopic fields per tumor, 2 tumors per mouse, 4-5 mice per treatment group). Pac: paclitaxel. PCat-siSur: PCat-siSurvivin. *, p<0.05 compared to vehicle control, single agent PCat-siNT or single agent PCat-siSurvivin groups. **, p<0.05 compared to all other paclitaxel-treated groups. ***p<0.05 compared to all other groups. White or black bars: 25 μm.