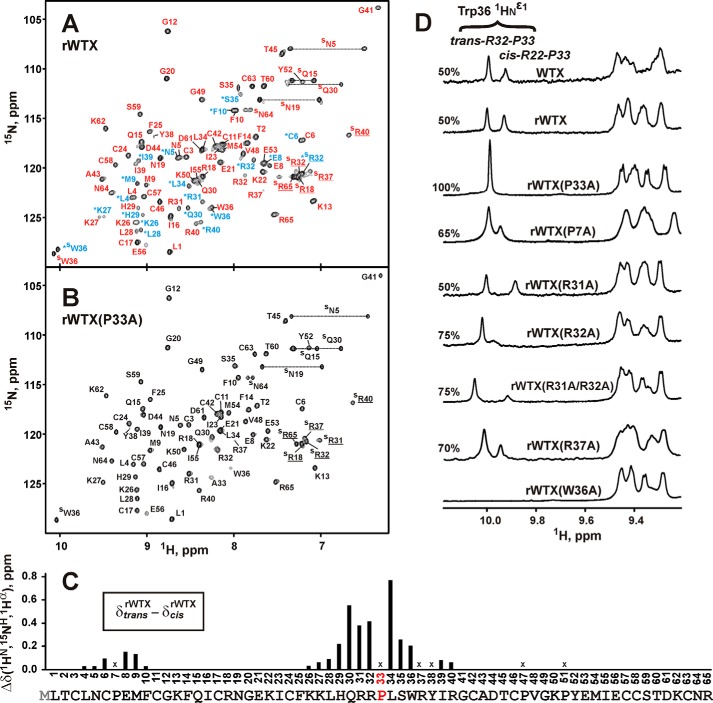
FIGURE 4.
Conformational heterogeneity in rWTX molecule associated with the cis-trans-isomerization of the Arg-32–Pro-33 peptide bond. A and B, two-dimensional 1H,15N-HSQC spectra of 0.5 mm rWTX and rWTX(P33A) (pH 3.0, 40 °C). Signals of rWTX conformers with trans- and cis-configuration of Arg-32–Pro-33 peptide bond are marked by red and blue lettering, respectively. The signals of the cis form are also marked by asterisks. C, normalized difference of 1HN, 1Hα, and 15NH chemical shifts (√(Δδ1HN)2 + (Δδ1Hα)2 + (Δδ15NH/5)2) between “trans” and “cis” forms of rWTX. The data for proline residues and for Arg-37 and Tyr-38 (crosses) were not calculated. Signals of Arg-37 and Tyr-38 residues from the trans form of rWTX were not assigned. D, analysis of conformational heterogeneity in rWTX mutants. The fragments of one-dimensional 1H NMR spectra containing HNϵ1 signals of the Trp-36 side chain are shown. The relative content (%) of the trans form is shown above each spectrum.