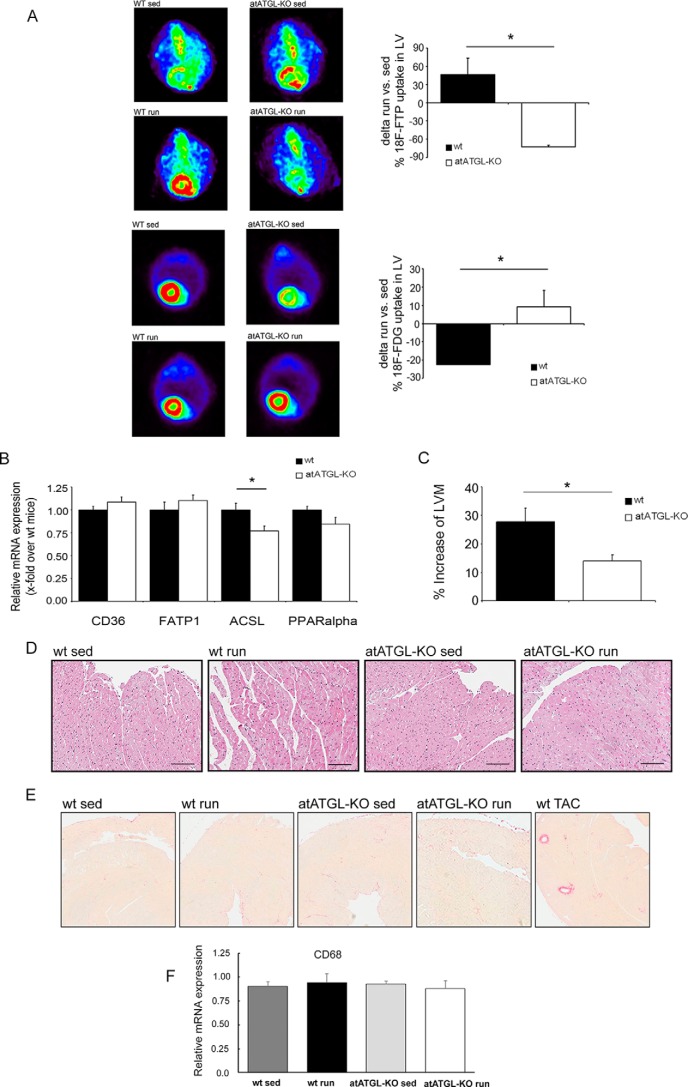
FIGURE 2.
Exercise-mediated changes in cardiac morphology/metabolism. A, representative images of the myocardial FA uptake ([18F]fluoro-4-thia-palmitate (18F-FTP)) (top 4 panels) and glucose uptake (2-[18F]fluorodeoxyglucose (18F-FDG) (bottom 4 panels), measured using small animal PET. Quantification of [18F]fluoro-4-thia-palmitate and 2-[18F]fluorodeoxyglucose PET, shown as percent change of mean sedentary myocardial uptake, was calculated as injection dose (%ID) relative to LVM (%ID/LVM) (n = 5; *, p < 0.05 versus WT (unpaired t test)). B, analysis of mRNA expression in LV tissue collected from trained mice immediately following their final running session, as a marker of lipid uptake and mitochondrial fatty acid oxidation. Quantitative RT-PCR studies were carried out using total RNA isolated from LV tissue. Data are presented as x-fold over WT mice. Cd36, Cluster of Differentiation 36; Fatp1, fatty acid transport protein 1; Acsl, acyl-CoA synthetase-1; Ppar-α, peroxisome proliferator-activated receptor α. *, p < 0.05 versus WT mice (n = 8; unpaired t test). C, cardiac adaptation expressed as percent change from mean sedentary LVM, as described previously (37). *, p < 0.05 versus WT mice (n = 9; unpaired t test). D, H&E -stained heart sections from WT and atATGL-KO mice. E, Picrosirius red-stained heart sections from WT and atATGL-KO mice (fibrosis), positive control (right image). LV samples were from mice with transverse aortic constriction (TAC) resulting in pathological cardiac hypertrophy. F, quantitative RT-PCR analysis of Cd68 mRNA expression levels measured in LVs of WT and atATGL-KO mice, as indicated.