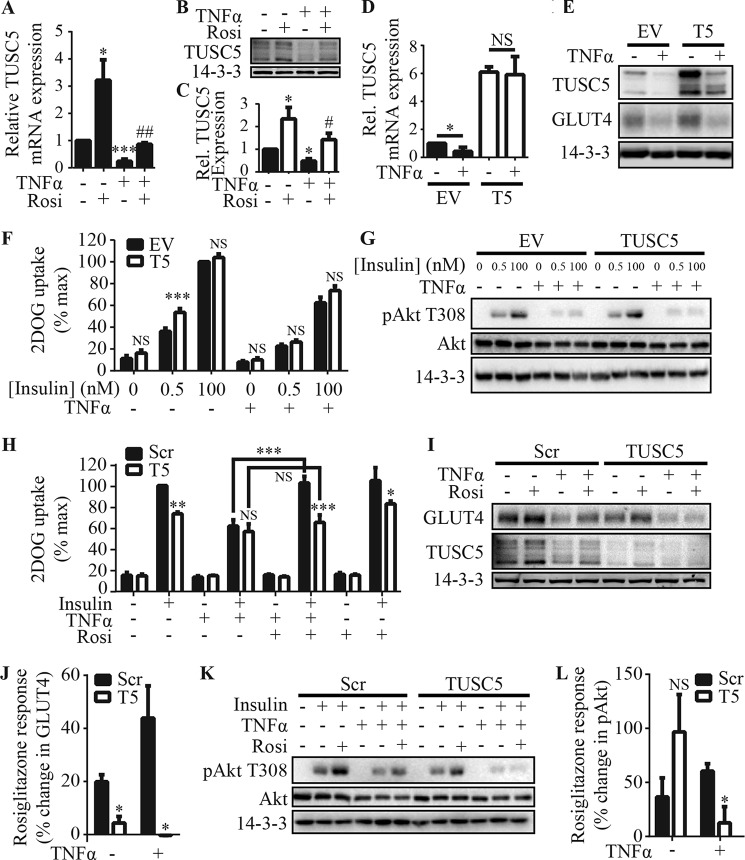
FIGURE 5.
TUSC5 is necessary, but not sufficient, for the insulin-sensitizing effects of rosiglitazone in insulin resistance. A, Tusc5 mRNA levels were determined by qPCR in cells treated with TNFα (2 ng/ml, 96 h) and/or rosiglitazone (10 μm, 48 h) as indicated. Data were expressed as relative to Tusc5 levels in control cells (n = 3, mean ± S.E., unpaired t test; *, p < 0.05; ***, p < 0.001 for comparisons with untreated cells; ##, p < 0.05 for comparisons with TNFα-treated cells). B, TUSC5 levels were determined by immunoblotting in cells treated with TNFα (2 ng/ml, 96 h) and/or rosiglitazone (10 μm, 48 h) as indicated. α-Tubulin levels were determined as a loading control. C, TUSC5 abundance (B) was quantified by densitometry and expressed as relative to untreated cells (n = 4, mean ± S.E., unpaired t test; *, p < 0.05 for comparisons with untreated cells; #, p < 0.05 for comparisons with TNFα-treated cells). D, Tusc5 mRNA levels were determined by qPCR in cells expressing an empty vector control (EV) or overexpressing TUSC5 (TUSC5) with and without TNFα treatment. Data were expressed relative to TUSC5 levels in untreated EV adipocytes (n = 3, mean ± S.E., unpaired t test; NS, nonsignificant; *, p < 0.05 for comparisons between untreated and TNFα-treated cells as indicated). E, TUSC5, GLUT4, and 14-3-3 (loading control) levels were assessed by immunoblotting whole cell lysates from cells expressing a control vector (EV) or overexpressing TUSC5 and treated with TNFα as indicated (representative of n = 4). F, 3T3-L1 adipocytes expressing a control vector (EV) or overexpressing TUSC5 were treated with TNFα (2 ng/ml, 96 h) as indicated before 2DOG uptake assays were performed. 2DOG uptake was expressed as a percentage of the maximum response (n = 4, mean ± S.E., two-way ANOVA; NS, nonsignificant; ***, p < 0.001; comparisons with cells expressing control vector (EV) under the same treatment conditions). G, 3T3-L1 adipocytes expressing a control vector (EV) or overexpressing TUSC5 were treated with TNFα (2 ng/ml, 96 h) as indicated before insulin signaling at the level of Thr(P)-308 AKT was monitored in response to 0.5 or 100 nm insulin (representative of n = 4). H, 3T3-L1 adipocytes were treated with scrambled or anti-Tusc5 siRNA for 96 h and treated with TNFα (2 ng/ml, 96 h) and/or rosiglitazone (10 μm, 48 h) as indicated before 2DOG uptake assays were performed in unstimulated cells or cells stimulated with 100 nm insulin. 2DOG uptake was expressed as a percentage of the maximum response (n = 5, mean ± S.E., two-way ANOVA; NS, nonsignificant; *, p < 0.05; **, p < 0.01; ***, p < 0.001, comparisons with control (scr) cells under the same treatment conditions unless otherwise indicated). I, GLUT4, TUSC5, and 14-3-3 (loading control) levels were assessed by immunoblotting whole cell lysates from control (scr) and TUSC5 knockdown cells treated with TNFα and/or rosiglitazone as indicated. J, quantification of the change in GLUT4 expression in response to rosiglitazone in cells treated with scrambled (Scr) or pooled anti-Tusc5 (T5) siRNA with and without TNFα treatment (n = 3, mean ± S.E., unpaired t test; *, p < 0.05, for comparisons with control (scr) cells under the same treatment conditions). K, 3T3-L1 adipocytes were treated with scrambled or anti-Tusc5 siRNA for 96 h and treated with TNFα and/or rosiglitazone as indicated before insulin signaling in response to 100 nm insulin was monitored at the level of Thr(P)-308 AKT (representative of n = 3). L, quantification of the change in insulin-stimulated phosphorylation of AKT at Thr-308 in response to rosiglitazone in cells treated with scrambled (Scr) or pooled anti-Tusc5 (T5) siRNA with and without TNFα treatment (n = 3, mean ± S.E., unpaired t test; NS, nonsignificant; *, p < 0.05 for comparisons with control (scr) cells under the same treatment conditions).