FIGURE 1.
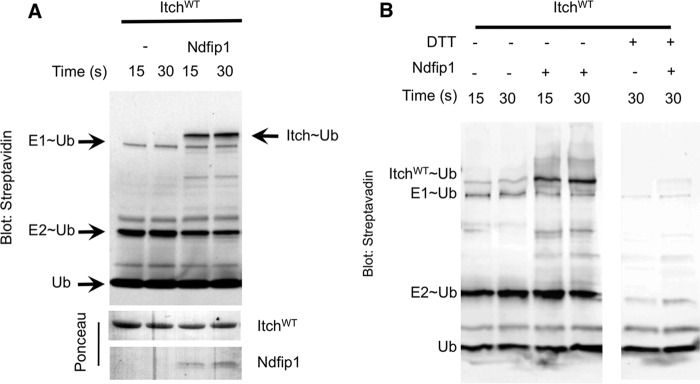
Ndfip1 promotes ubiquitin charging of Itch. A, Itch was incubated for the indicated time points with (biotinylated) ubiquitin-loaded E2 and in the presence or absence of Ndfip1. Streptavidin blotting revealed ubiquitin-charged Itch. Ponceau staining was used as a loading control. B, samples from the Itch charging experiment shown in A were divided into two tubes, and one tube was treated with 50 mm DTT. DTT treatment resulted in loss of ubiquitin charging on Itch, indicating that the interaction was mediated by the characteristic thioester bond between the C-terminal glycine tail of ubiquitin and the catalytic cysteine in the HECT domain of Itch. In all panels, similar results were obtained in at least three independent experiments.
