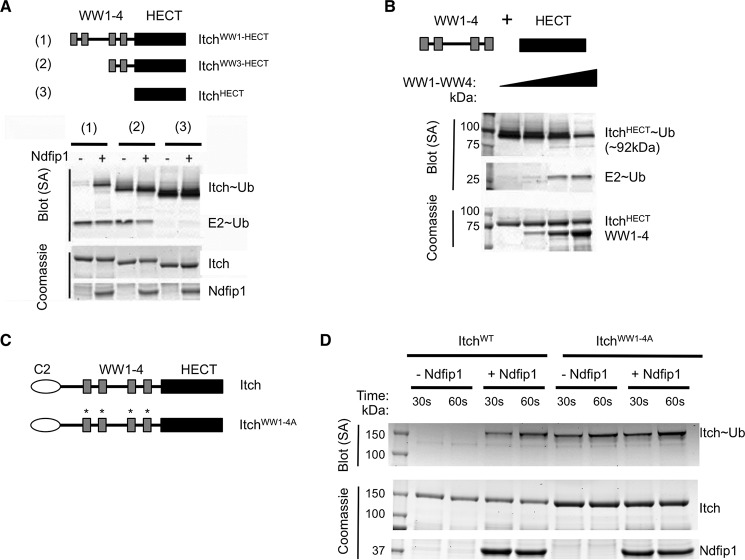
FIGURE 4.
Itch autoinhibition is due to an intramolecular interaction between its WW and HECT domains. A, constructs were generated that lack two or all four WW domains, as illustrated. Constructs were assessed for autoinhibition using the ubiquitin charging assay. Streptavidin shows ubiquitylated proteins, and Coomassie shows loading controls. B, cassettes containing all four WW domains or the HECT domain were generated as depicted. Ubiquitin charging of the HECT domain was assessed in the presence of increasing concentrations of the WW domain cassette (His-MBP-HECT is ∼84.2 kDa). Again, streptavidin shows ubiquitylated proteins, and Coomassie shows loading controls. C, full-length Itch and a construct in which the following point mutations were made in the binding pocket of each WW domain: W315A, W347A, W427A, and Y467A (indicated by asterisks and, together, termed ItchWW1–4A). D, these constructs were analyzed for their ability to accept ubiquitin in the presence or absence of Ndfip1 using the ubiquitin charging assay. In A, B, and D, similar results were obtained in at least three independent experiments. SA, streptavidin.