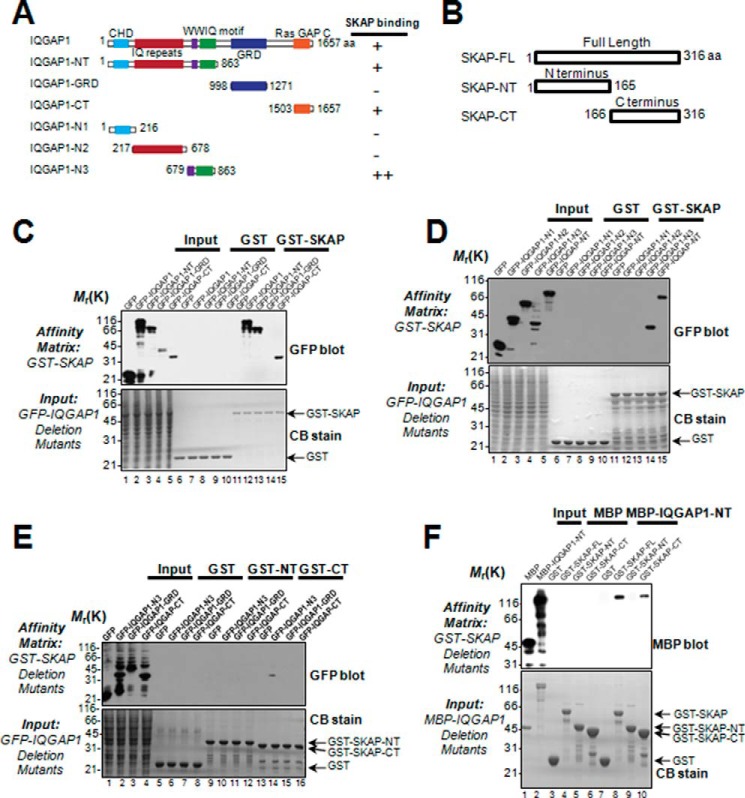
FIGURE 2.
Biochemical characterization reveals a physical link between the SKAP C terminus and IQGAP1-NT. A, schematic drawing of IQGAP1 truncation mutants. Residue numbers at domain boundaries are indicated. B, schematic drawing of SKAP truncation mutants. Residue numbers at domain boundaries are indicated. C, SKAP binds to the IQGAP1 N (amino) terminus as well as C (carboxyl) terminus. Purified GST-SKAP proteins were used to isolate GFP-IQGAP1 deletion mutant proteins from HEK293T cell lysates and fractionated by SDS-PAGE (lower) followed by anti-GFP blotting analysis (upper). D, purified GST-SKAP proteins were used to isolate GFP-IQGAP1 N-terminal truncations from HEK293T cell lysates. Western blotting analysis with anti-GFP antibody indicated the specific associations. E, purified GST-SKAP truncations were used to absorb GFP-IQGAP1 truncations from HEK293T cell lysates. Western blotting analysis with anti-GFP antibody indicated the specific association. F, SKAP directly binds to scaffolding protein IQGAP1 via its C terminus. Using purified GST-SKAP truncations as matrices, a GST pulldown assay was performed to bind purified MBP-tagged IQGAP1-NT. Western blotting analysis with anti-MBP antibody confirmed a specific interaction.