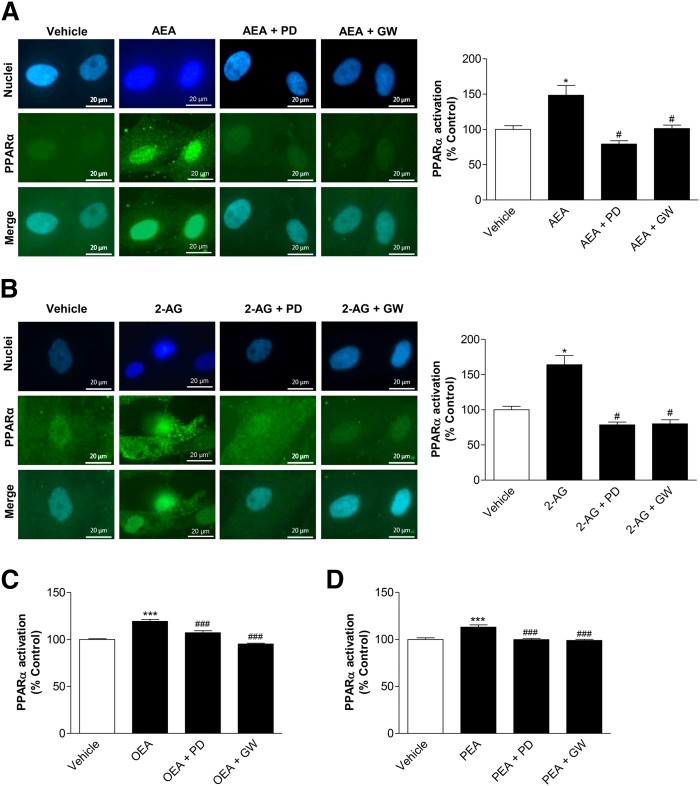
Fig. 7.
Impact of p42/44 MAPK on PPARα activation by endocannabinoids and endocannabinoid-like substances. A, B: MSCs were pretreated with the upstream inhibitor of p42/44 MAPK activation, PD98059 (PD) (10 μM), or the PPARα antagonist, GW6471 (GW) (10 μM), for 1 h and subsequently incubated with AEA (A, 10 μM) or 2-AG (B, 10 μM) for another 2 h. PPARα activation was quantified by measuring colocalization of PPARα and nuclear regions of MSCs. Nuclear regions were visualized by the DNA intercalating fluorescent dye, bisbenzimide. Nuclear PPARα was quantified by merging nuclear regions with PPARα fluorescence intensity. Pictures at the left show representative images of nuclear regions (blue), PPARα (green), and PPARα merged with nuclei, respectively. C, D: Experiments for evaluation of the influence of PD98059 and GW6471 on OEA-induced (C) or PEA-induced (D) PPARα activation were carried out using the same protocols as (A) and (B). For quantitiative evaluation of nuclear PPARα, fluorescence at 488 nm excitation of n = 40–43 (A), n = 13–18 (B), n = 46 (C), or n = 24 (D) nuclei was measured using the Zeiss Zen Pro 2012 analysis software. Percent control represents PPARα fluorescence in nuclear regions indicated as mean ± SEM in comparison to vehicle-treated cells (100%) in the absence of test substances using cells obtained from one donor. *P < 0.05 versus vehicle control; #P < 0.05 versus the respective endocannabinoid (A, B) or endocannabinoid-like substance (C, D) in the absence of antagonists, one-way ANOVA plus post hoc Bonferroni test.