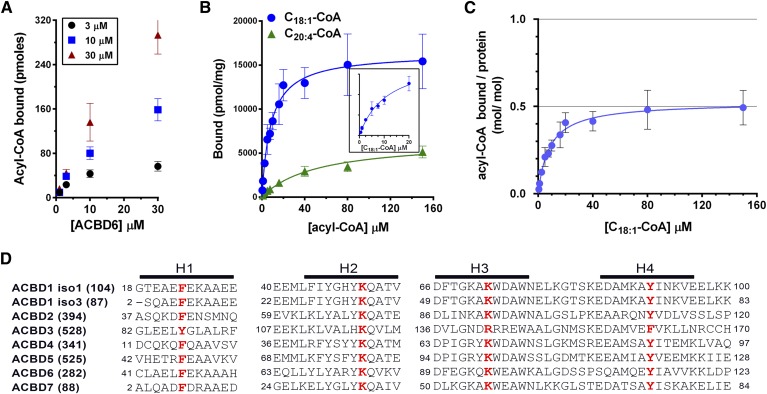
Fig. 1.
Acyl-CoA binding activity of human ACBD6. A: Three different concentrations of [14C]C18:1-CoA were incubated with increasing concentrations of purified ACBD6 at 37°C for 20 min in 100 µl of 10 mM potassium phosphate pH 7.4. Unbound ligand was separated from ACBD6-bound ligand by pulling down the hexahistidine-tagged protein with NTA resin. B: Increasing concentrations of [14C]C18:1-CoA (blue circles) and [14C]C20:4-CoA(green triangle) were incubated with 1 µM ACBD6. Inset: Data of the binding of [14C]C18:1-CoA were plotted for the concentration range of 0 to 20 µM. C: Molar ratio of [14C]C18:1-CoA bound per mole of ACBD6 was plotted as function of the concentration of ligand. Errors represent the standard deviations of three measurements. D: Amino acid alignment of the predicted four helices (H1 to H4) of the ACB motif of the seven members of the ACBD family. For clarity, DBI was annotated as ACBD1 and PECI as ACBD2 in the panel. Two of the several spliced variants of ACBD1 are shown (isoform 1 and isoform 3). The number of residues of each form is indicated in parentheses. The four conserved residues predicted to be essential for acyl-CoA binding that were substituted with alanine are shown in bold red.