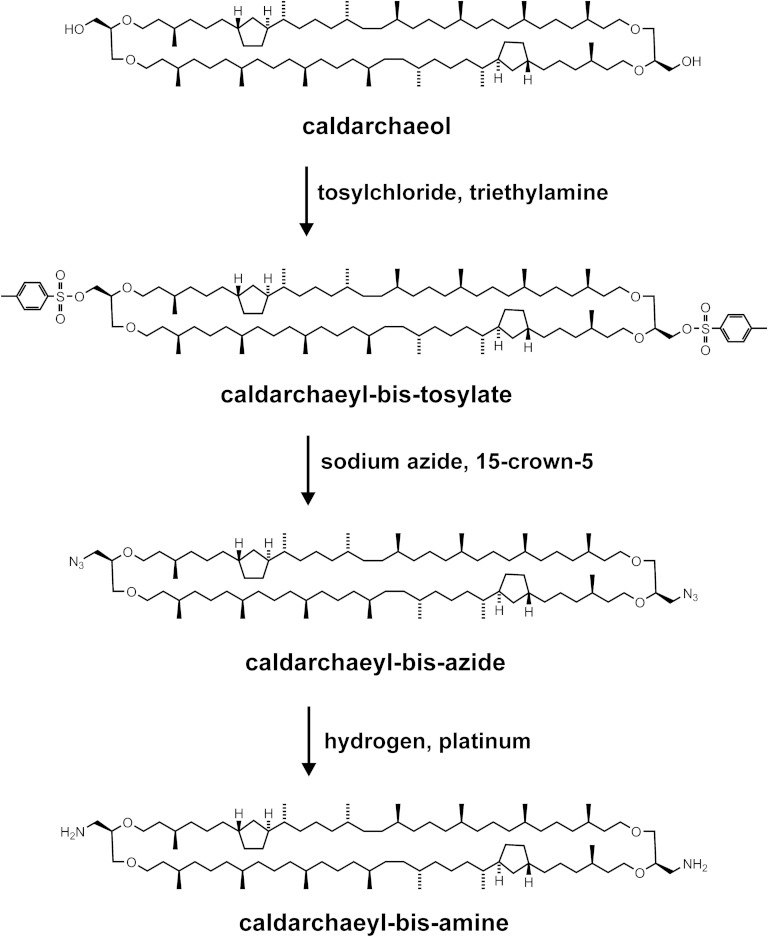
Fig. 4.
Synthetic route to MSLs with fluorescent tags directly tied to the tetraether lipid core. Caldarchaeol reacted with tosyl chloride in the presence of triethylamine to give caldarchaeyl-bis-tosylate, which in the presence of the crown ether 15-crown-5 was transformed into caldarchaeyl-bis-azide. This azide was subjected to hydrogen in the presence of platinum to yield caldarchaeyl-bis-amine as a precursor for fluorescent MSL derivatives without a phosphoethanolamine spacer. So far a green and a red fluorescent derivative were obtained by reacting caldarchaeyl-bis-amine with amine-reactive fluorophores whose structures are shown in Fig. 5B.