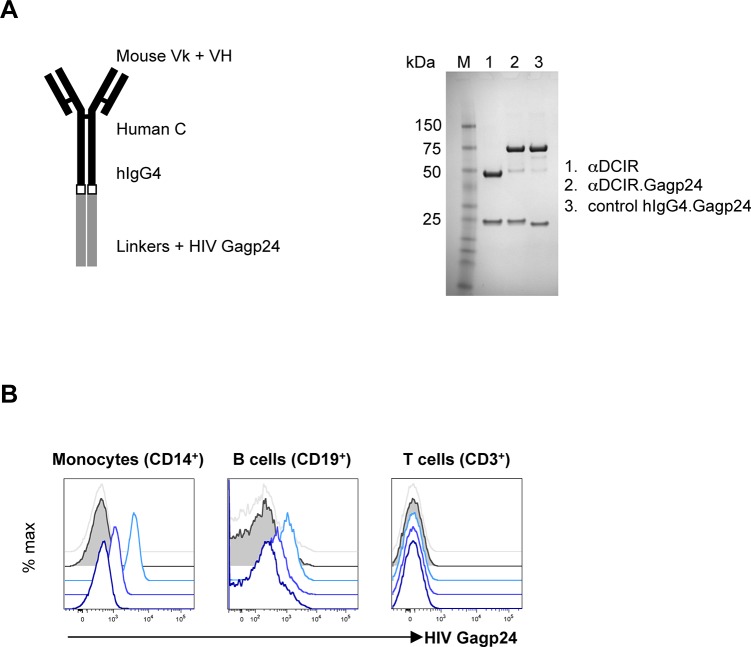
Fig 1. Characterization of the αDCIR.Gagp24 fusion rAbs.
A. Schematic representation and SDS-PAGE analysis under reducing conditions of the αDCIR.Gagp24 fusion protein. Expression constructs for chimeric mouse variable (V) region-human IgG4 constant (C) region αDCIR and isotype control recombinant antibodies (rAbs) were engineered with the HIV Gagp24 coding region fused in frame to the heavy (H) chain C-terminus (left panel). These constructs were co-transfected with a matching light (k) chain expression vector into stable CHO-S cell lines and the secreted fusion rAbs were purified from the culture supernatants by protein A-affinity chromatography and analyzed on reducing SDS-PAGE. Proteins were stained with Coomassie Blue (right panel). B. Specific binding of αDCIR.Gagp24 rAb. Monocytes (left panel), B cells (middle panel) and T cells (right panel) from an HIV-infected patient PBMCs were treated with 3 nM, 0.3 nM and 30 pM of αDCIR.Gagp24 and 3 nM hIgG4.Gagp24 fusion proteins, followed by incubation with an anti-HIV Gagp24 PE-conjugated antibody and then analyzed by flow cytometry. Blue traces are staining from αDCIR.Gagp24. The blue color gradient represents the different concentrations from light blue (3 nM) to dark blue (30 pM). Grey traces (staining by 3 nM hIgG4.Gagp24) and grey solid traces (untreated cells) are the background staining. Data are representative of 2 different patients.