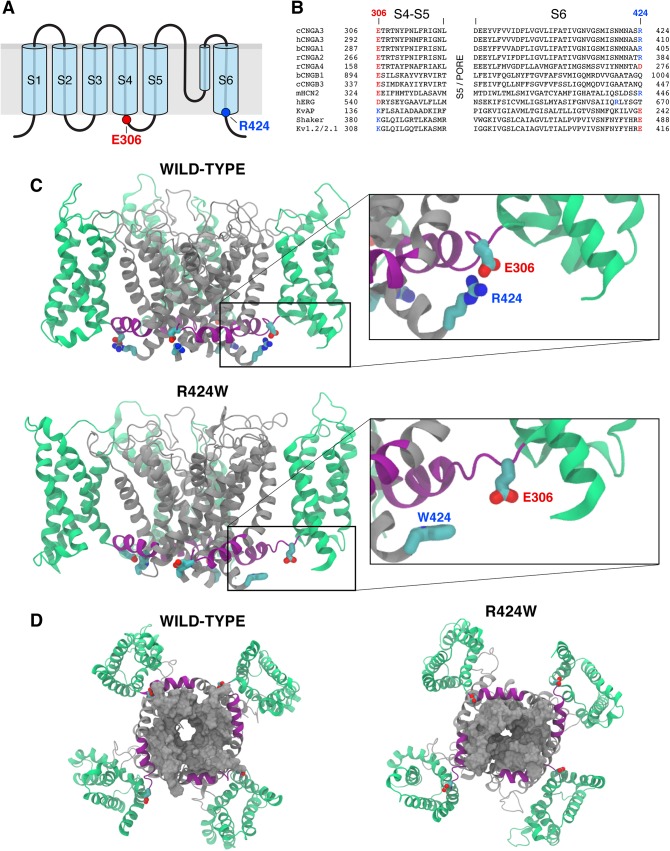
Fig 2. R424W mutation disrupts salt bridge interaction and destabilizes the open state of pore in a homotetrameric CNGA3 model.
(A) Schematic representation of CNGA3 subunit consisting of six transmembrane (TM) spanning segments (S1-S6) and a pore domain between S5 and S6. The highlighted last residue of S6 (blue) is the site of canine CNGA3-R424W mutation; its predicted partner, glutamic acid E306, is the first residue of S4-S5 linker. (B) Amino acid sequence alignment of the S4-S5 linker and S6 segment of selected shaker K+ channel superfamily members. The TM regions of the CNG channel family were assigned using the crystal structure of the chimeric voltage-gated potassium channel Kv1.2/2.1 (PDB ID: 2R9R). Sequence alignments of S5 domain and pore region were omitted for clarity. The R424 residue is shown in blue and its interacting partner, E306 in red. The conserved salt bridges in the Kv channels show opposite charges at these positions. c = canine, b = bovine, h = human, r = rat, m = mouse. (C) Side view of the wild-type CNGA3 homotetramer model and the CNGA3-R424W mutant channel equilibrated in its environment. The voltage-sensing domain (S1-S4) is presented in green, the S4-S5 linker in purple and the pore-forming region (S5-S6) in grey. The residues E306 and R424 are shown as red and blue rods, respectively. The E306:R424 interaction (wild-type) or its loss (R424W mutant) is demonstrated on the higher magnification images. Carbon atoms are labeled in cyan, nitrogens in blue and oxygens in red. Other side chains were omitted for clarity. Note that R424 forms a salt bridge with the E306 molecule in three subunits out of four. (D) Bottom views of the wild-type CNGA3 and CNGA3-R424W mutant channels. S6 is represented as a grey solid surface highlighting the partial closure of the pore in the R424W mutant model.