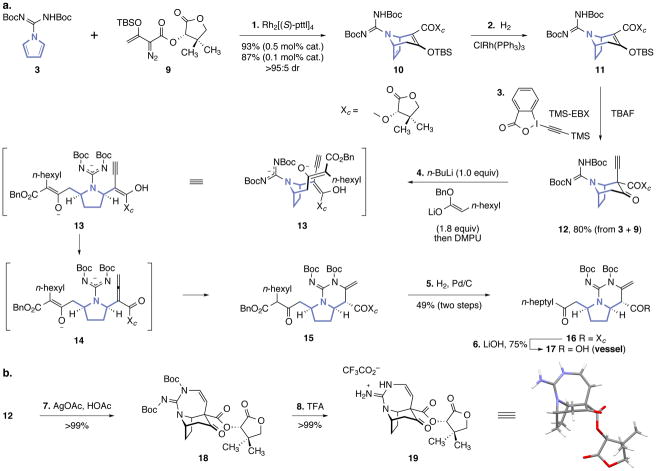
Figure 2. Synthesis of the vessel fragment of (+)-batzelladine B (1) and determination of stereochemistry.
a, Synthesis of the vessel precursor 17. Reagents and conditions: 1. Rh2[(S)-pttl]4 (0.5 mol%), pentane, 36 °C, 93%, >95:5 dr, or Rh2[(S)-pttl]4 (0.1 mol%), pentane, 36 °C, 87%, >95:5 dr. 2. H2 (30 atm), ClRh(PPh3)3 (2.0 mol%), i-PrOH, 23 °C. 3. TBAF, TMS-EBX, THF–CH2Cl2 (8:1), −78 °C, 80% (from 3 + 9). 4. n-BuLi, then lithium benzyl octanoate, THF, then DMPU, −78 °C. 5. H2 (1 atm), Pd/C (10 mol%), THF, 23 °C, 49% (two steps). 6. LiOH, THF–H2O (2:1), 0 °C, 75%. b, The relative stereochemistry of 12 was established by cyclization and deprotection, followed by X-ray analysis. Reagents and conditions: 7. AgOAc, AcOH, CH2Cl2, 24 °C, >99%. 8. TFA, CH2Cl2, 0→23 °C, >99%.