Figure 3. Synthesis of the (+)-batzelladine B (1) anchor.
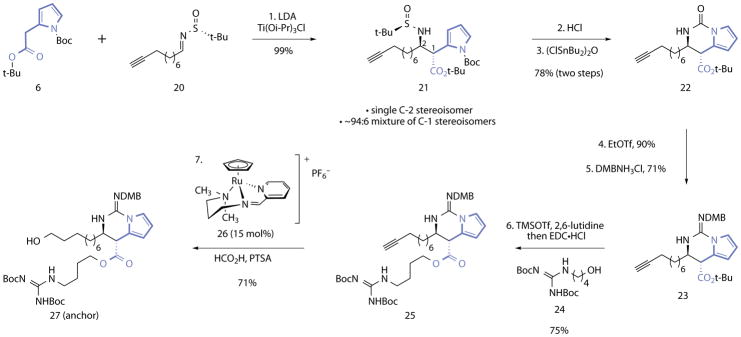
Reagents and conditions: 1. LDA, Ti(Oi-Pr)3Cl, THF, −78 °C, 99%, >20:1 mixture of C-1 stereoisomers, ~94:6 mixture of C-2 stereoisomers. 2. HCl, CH3OH–1,4-dioxane (4.4:1), 0 °C. 3. (ClSnBu2)2O, toluene, 100 °C, 78% (two steps). 4. EtOTf, 2,4,6-tri-tert-butyl-pyrimidine, CH2Cl2, 23 °C, 90%. 5. DMBNH3Cl, 3 Å MS, EtOH, 70 °C, 71%. 6. TMSOTf, 2,6-lutidine, CH2Cl2, 0→23 °C, then 24, DMAP, EDC•HCl, CH2Cl2, 0→23 °C, 75%. 7. 26 (15 mol%), PTSA (1.0 equiv), HCO2H, NMP–H2O (4:1), 23 °C, 71%.
