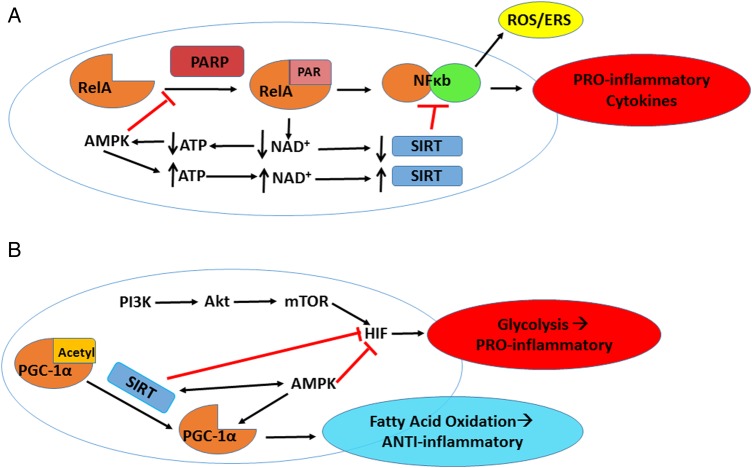
Figure 2.
Poly(ADP-ribose) polymerase (PARP) and sirtuin (SIRT) effects on immunometabolism. A, PARP “PARylates” many substrates including RelA for activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in an oxidized nicotinamide adenine dinucleotide (NAD+)–dependent manner, leading to elevated reactive oxygen species (ROS)–oxidative stress/endoplasmic reticulum stress (ERS) and proinflammatory cytokine production. PARP hyperactivity also causes chronic inflammation by depleting NAD+ that subsequently decreases SIRT activity and lowers adenosine triphosphate (ATP) concentrations. SIRT can counteract NF-κB activation through deacetylation of the RelA/p65 subunit of NF-kB. Depleted ATP activates AMP-activated protein kinase (AMPK), which in turn increases ATP and NAD+ levels, leading to increased SIRT activity and suppressed PARP activity that may bring the cell back to equilibrium. B, SIRT regulates immune cell metabolic activity largely through regulation of hypoxia-inducible factor (HIF1α) and peroxisome proliferator–activated receptor γ coactivator (PGC-1α). Increased phosphatidylinositol 3-kinase (PI3K)/serine-threonine protein kinase (AKT)/mechanistic target of rapamycin (mTOR) signaling activates HIF to convert cell energy production mechanism to aerobic glycolysis during immune activation enhancing T-helper 1 cell differentiation. SIRT, along with AMPK, converts T-cell energy metabolism to fatty acid oxidation by blocking HIF activity and activating PGC-1α to enhance differentiation toward memory T cells and T-regulatory cells.