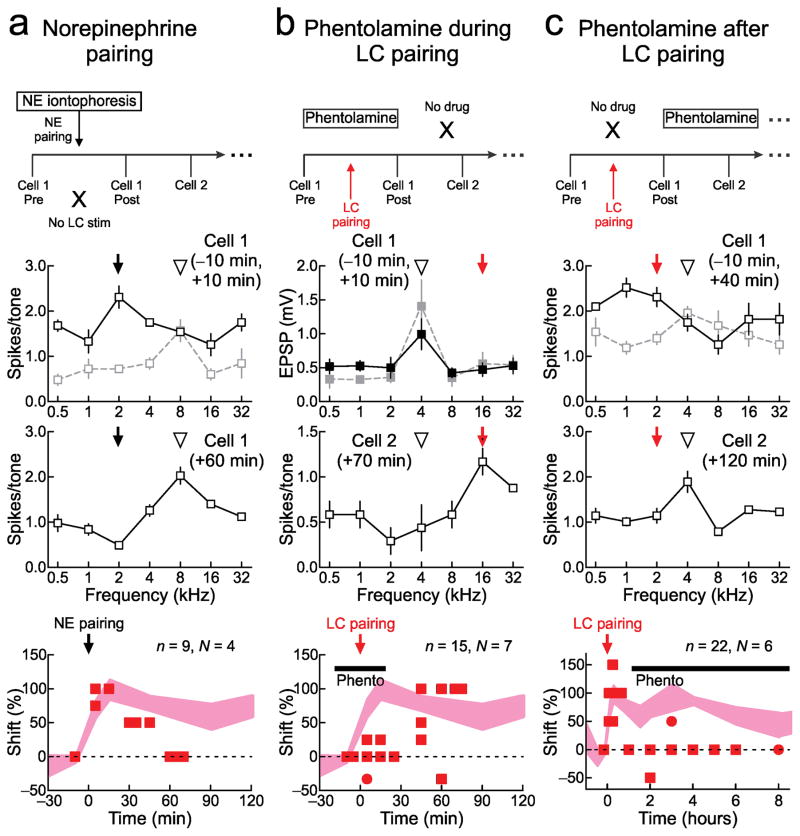
Figure 6.
Noradrenergic receptor activation is required for expression of AI plasticity. a, Norepinephrine pairing (‘NE pairing’) leads to shorter-term but not sustained AI changes. Top, experimental design; pure tones were paired with NE iontophoresis (0.1 mM) in AI. Middle, cell-attached recording before and after NE pairing with 2 kHz (arrow). Initially, best frequency (open arrowhead) shifted from 8 kHz to 2 kHz, but returned to 8 kHz one hour later. Bottom, NE pairing summary (best frequency shift 10 minutes post-pairing: 95.8±5.1%, n=4, N=4, P=10−5; shift 45+ minutes post-pairing: 8.3±10.2%, n=4, N=4 animals, P=0.3). b, Cortical phentolamine (0.1–1 mM) only during LC pairing prevents shorter-term changes, but longer-term changes emerge when phentolamine is removed. Top, experimental design. Middle, whole-cell recording before and after pairing, and cell-attached recording one hour post-pairing. Original best frequency was 4 kHz, tuning was unchanged 10 minutes post-pairing in presence of phentolamine (0.1 mM), but shifted to paired 16 kHz tone one hour later when phentolamine was removed. Bottom, phentolamine during pairing summary (shift 10 minutes post-pairing: 1.5±62%, n=6, N=6, P=0.7; shift 45+ minutes post-pairing: 67.8±17.7%, n=8, N=7, P=0.0006). c, Phentolamine applied after pairing for hours shortens AI shift duration. Top, experimental design-phentolamine (0.1 mM) was applied to AI for subsequent recordings after pairing. Middle, two cell-attached recordings before and after LC pairing. Bottom, phentolamine after pairing summary (shift 10 minutes post-pairing: 85.0±10.7%, n=6, N=6, P=10−4; shift 3–8 hours post-pairing: 6.3±6.3%, n=8, N=5, P=0.3). All comparisons with unpaired two-tailed t-tests. Error bars indicate s.e.m.