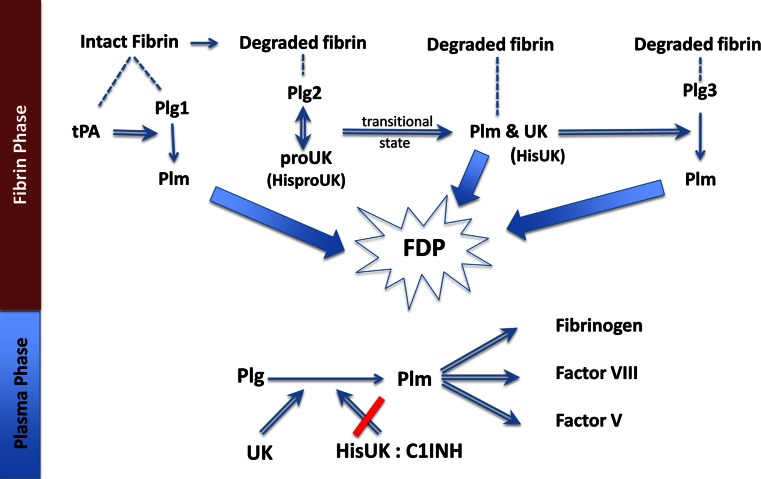
Fig. 1.
Fibrinolysis by the sequential modes of action of tPA and proUK or HisproUK: tPA binds (dashed line) to intact fibrin adjacent to plasminogen(Plg1), forming a ternary complex and activating Plg1 to plasmin (Plm). This initiates fibrin degradation resulting in the creation of new plasminogen binding sites on fibrin. Plg2 binds to a high-affinity binding site on the fibrin E (FFE) domain, which induces a special conformational shape change in Plg2 that enables it to be activated by the intrinsic activity of HisproUK. This HisproUK:plasminogen complex results in Plm formation and the reciprocal activation (double arrow) of HisproUK → HisUK, associated with a transitional state that is hypercatalytic. UK, being a non-specific enzyme, then activates the remaining fibrin-bound plasminogen (Plg3). The three Plm together complete fibrin degradation (thick arrows) forming soluble fibrin degradation products (FDP). In the Plasma Phase, when UK diffuses off the fibrin clot, if it is not inhibited, it will activate plasma Plg to Plm, resulting in the degradation of 3 clotting factors, among other side effects. When HisproUK is used in place of proUK, this non-specific effect is prevented due to HisUK being inhibited by C1INH, whose plasma concentration is >1000-fold greater than that of the UK inhibitor