Abstract
Purpose
Histotripsy employs pulsed cavitational ultrasound for non-invasive tissue ablation. Some forms of therapeutic ultrasound cause intravascular hemolysis. We investigated the extent and consequences of histotripsy induced hemolysis in vivo.
Materials and Methods
Porcine femoral venous blood was treated with histotripsy in 11 animals with systemic heparinization and 11 without heparin. Serum and hemodynamic measurements were obtained at 0, 2, 5, 10, 15, 30 minutes, and 48–72 hours post-procedure. Fischer’s exact test was used to determine differences in mortality between heparinized and non-heparinized groups. A linear mixed effects model was used to test for differences in blood-analytes and hemodynamic variables over time.
Results
Of 11 non-heparin treated animals, 5 died during or immediately following histotripsy (non-heparin mortality 45% vs. heparin mortality 0%, p=0.035). Serum hematocrit, free hemoglobin, LDH, and right ventricular systolic pressure (RVSP) changed significantly (p<0.001) over the treatment time. Serum hematocrit decreased slightly (32.5 ± 3.6 to 29.4 ± 4.2%), while free hemoglobin (6.2 ± 4.6 to 348 ± 100 mg/dL), LDH (365 ± 67.8 ± to 722 ± 84.7 U/L), and RVSP (23.2 ± 7.2 to 39.7 ± 12.3 mmHg) increased. After 48 to 72 hours, hematocrit remained slightly decreased (p=0.005), while LDH and free hemoglobin remained slightly increased compared to baseline (both p<0.001).
Conclusion
Intravascular histotripsy applied to free flowing venous blood is safe with systemic heparinization, causing only transient hemodynamic and metabolic disturbances, thereby supporting its use as a future non-invasive thrombolytic therapy modality.
Introduction
Pulsed cavitational ultrasound, or histotripsy, discretely and non-invasively mechanically fractionates tissue (1). Animal studies have established several potential clinical applications, including deep vein thrombosis treatment, creation of palliative intracardiac communications in infants with congenital heart disease, and in utero palliation of congenital malformations (2–4). Other forms of therapeutic ultrasound with thermal effects cause hemolysis with increasing therapeutic intensity (5). Unlike these other modalities, histotripsy utilizes short, high intensity pulses to mechanically fractionate tissue through acoustic cavitation, and without thermal necrosis (1). Nevertheless, hemolysis remains a concern as in vitro studies have suggested acoustic cavitation may cause hemolysis in the absence of thermal effects (6).
While the safety profile of histotripsy has been investigated generally, the effect of histotripsy on the circulating blood volume with regards to hemolysis has not been previously described. Determining the extent of hemolysis following histotripsy is clinically important, as hemolysis can lead to acute anemia. Additionally, hemolysis increases serum free hemoglobin, potentially leading to endothelial dysfunction and secondary platelet activation and aggregation, renal injury, intravascular thrombosis and pulmonary hypertension (7).
The purpose of this study was to establish the safety profile of histotripsy with respect to the circulating blood volume, primarily by evaluating reduction in serum hematocrit following histotripsy as a marker for clinically significant hemolysis. Secondary objectives included evaluation of clinically relevant sequelae of hemolysis, such as hyperkalemia, acute kidney injury, and pulmonary hypertension.
Materials and Methods
Animal Model
The protocols described herein were approved by the University Committee on Use and Care of Animals at our institution. A porcine model was developed that maximizes the potential adverse effects of histotripsy, including hemolysis, on the circulating blood volume in vivo by focusing therapy on free flowing blood through a femoral vein. Prior studies in our laboratory evaluating histotripsy mediated thrombolysis and the creation of intracardiac defects routinely employed systemic heparinization during therapy (2,3). As the target in the present study was not intracardiac tissue nor intravascular thrombus, we opted not to systemically heparinize half of the study animals, as the risk for thrombosis or embolization was thought to be low. However, because heparin has been shown to mitigate endothelial cell dysfunction, we also sought to ameliorate potential adverse effects of histotripsy by administering heparin to half the animals (8,9). As the precise effects of heparinization during histotripsy were unknown, sample size was determined based on statistical power to detect a hypothesized higher mortality rate in the non-heparin group. With 11 pigs per group, there was 82% power to detect a > 30% increase in mortality in the non-heparinized group.
Animal Preparation
Animal preparation and the histotripsy therapy apparatus used with this technique have been previously described in detail (2). Juvenile pigs (mixed breed and pure breed) weighing between 30–40 kg were sedated with 5 mg/kg teletamine + zolazepam (Telazol, Fort Dodge Animal Health, Fort Dodge, IA, USA) and 2.2 mg/kg xylazine (Lloyd Laboratories, Shenandoah, IA, USA), followed by endotracheal intubation and rotation to a supine position. Isoflurane 0.5–3.5% (Vet-One, Meridian, USA) was administered through the endotracheal tube for anesthesia. Animals were spontaneously breathing, though mechanical ventilation was initiated for those that became apneic during histotripsy. A chemical depilatory (Nair, Church & Dwight Co., Princeton, NJ, USA) was applied to the legs for 10 minutes for hair removal to provide adequate ultrasound transmission.
Using sterile technique, a 6 French sheath (Cook Medical, Bloomington, IN, USA) was percutaneously inserted into the right internal jugular vein or right femoral vein. A 5 or 6 French balloon wedge catheter (Arrow International, Reading, PA, USA) was advanced through the venous sheath and into the right ventricle. A 5 French micropuncture introducer (Cook Medical, Bloomington, IN, USA) was percutaneously inserted into the right femoral artery. Pressures from the right ventricle catheter and right femoral artery introducer were transduced simultaneously.
A hole was cut in the middle of a transparent polyethylene sheet, and Ioban (3M, St. Paul, MN, USA) was affixed over this hole. The Ioban was then affixed to the skin overlying the left femoral vein and a section of Ioban over the histotripsy target was cut away. The polyethylene sheet was then used to line a bottomless bowl, and the bowl was filled with warm, degassed water to minimize pre-focal cavitation. The use of this setup enabled direct ultrasound transmission to the skin overlying the femoral vein target, while ensuring an impermeable water bath sufficiently large to appropriately treat the target, given the fixed, 9 cm focal length of the therapy transducer (Figure 1).
Figure 1.
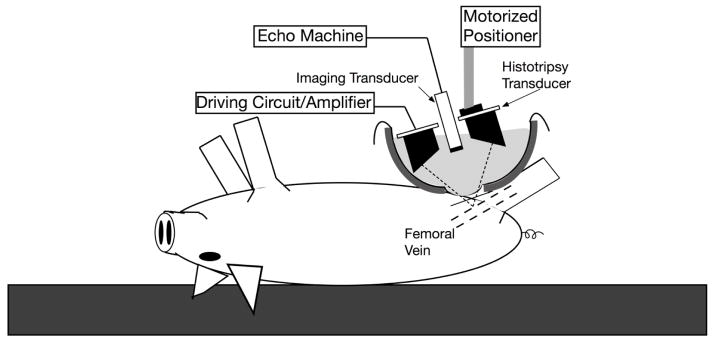
Schematic demonstrating the histotripsy therapy transducer and imaging transducer suspended over a water bath, oriented for femoral vein targeting
A 1 MHz 10 cm diameter spherically-focused transducer (Imasonic, Besancon, France) was attached to a computer-controlled 3-axis motorized positioning stage (Parker-Hannafin, Rohnert Park, CA, USA). The transducer had a 4 cm concentric hole, through which an 8 MHz phased array ultrasound imaging probe was placed (S8, SONOS 7500, Philips Healthcare, Andover, MA, USA). The 8 MHz probe was used to align the therapy focus within the femoral vein and provide real time visualization of histotripsy therapy.
Targeting, Treatment and Evaluation
The focus of the therapy transducer was located on the ultrasound image by applying histotripsy pulses to an empty water bath. The center of the bright, percolating cavitation cloud was marked as the focal position (Figure 2). Next, the focus was aligned within the left femoral vein. The 11 anticoagulated animals received 100 Units/Kg of unfractionated heparin (Sargent Pharmaceuticals, Schaumburg, IL). Additional heparin was given before starting histotripsy, if necessary, to maintain ACT > 200 seconds. The histotripsy transducer was driven to emit 5 cycle ultrasound pulses at 500 Hz for 30 minutes. The lowest transducer output necessary to produce a consistent bubble cloud was used for each treatment (Figure 3). These histotripsy transducer settings were selected, as the efficacy of this experimental setup has been previously demonstrated for thrombolysis of acute deep vein thrombosis in the femoral vein in a porcine model (2). Serum lab studies and hemodynamic values were obtained at baseline, and 2, 5, 10, 15, and 30 minutes following histotripsy initiation. For those animals that received heparin, a post-histotripsy ACT was checked and anticoagulation was reversed with protamine (APP Pharmaceuticals, Schaumburg, IL) if the ACT remained greater than 200 seconds. Repeat serum specimens were obtained 48 to 72 hours after therapy.
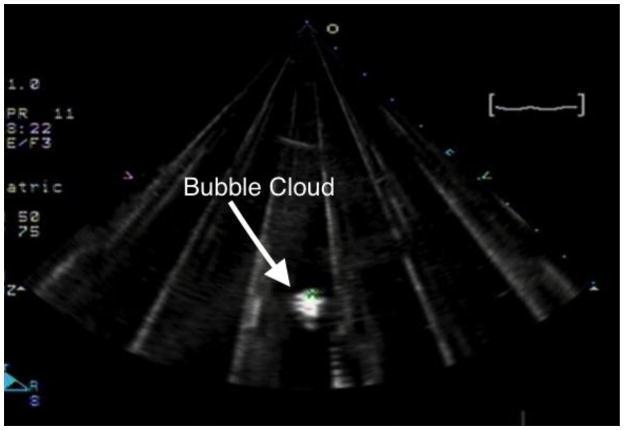
Figure 2.
A percolating histotripsy bubble cloud is induced in an empty water bath and is marked to subsequently assist in appropriately targeting the femoral vein.
Figure 3.
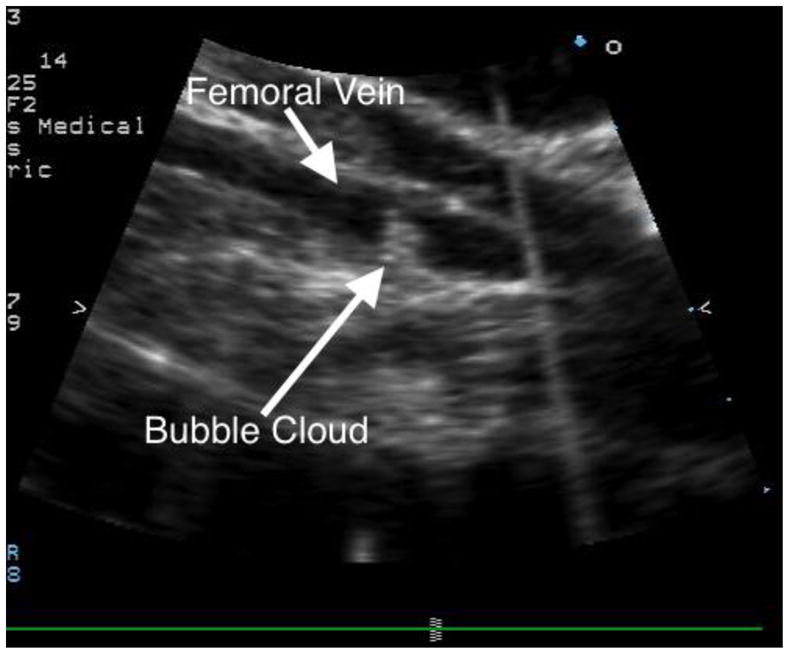
Histotripsy applied to the femoral vein lumen, as demonstrated by the echobright cavitation cloud. The femoral vein is imaged along its long axis.
Statistical Analysis
Fisher’s exact test was used to test for differences in frequency of death between heparinized and non-heparinized groups. The continuous blood-analyte variables were expressed as mean ± standard deviation. A linear mixed effects model was used to test for differences between treatment groups in blood-analytes over time (in minutes), where the within subject correlation structure was modeled using a linear term for time. A backwards model selection procedure was applied to the analysis of each blood analyte, with variables for the heparin treatment, linear and quadratic time in minutes, and the pairwise interaction terms between treatment and time included in the initial full model. Starting with the pairwise interactions, p-values were computed using likelihood ratio tests, and non-significant variables were successively removed from the model. Using a one-degree of freedom likelihood ratio test, the treatment effect was tested in the final model, which retained the treatment effect along with significant time and/or time-by-treatment interactions. P-values less than 0.05 were considered statistically significant. Except where otherwise noted, statistical analyses were carried out using the R statistical programming language (version 3.0.2).
Results
Mortality
All animals that received heparin therapy (11/11) completed the 30 minutes of histotripsy treatment versus only 7 out of 11 animals without heparin therapy. The four non-survivors developed apnea, hypotension, and ST segment changes immediately after therapy initiation and died during histotripsy treatment despite starting mechanical ventilation with the onset of apnea. The median duration of histotripsy in these animals was 3.5 minutes (range 2 to 5 minutes). Another animal in the non-heparin group developed apnea and required mechanical ventilation but completed 30 minutes of histotripsy. However, this animal could not be weaned from mechanical ventilation and was euthanized following histotripsy. In the heparin group, 3 animals developed apnea 8.5 to 21 minutes after starting histotripsy and briefly required mechanical ventilation. All 3 pigs were promptly weaned from the ventilator after completing 30 minutes of histotripsy. Overall mortality in the heparin treated group was 0%, versus 45% in the non-heparin group (p=0.035).
Immediate Histotripsy Effects
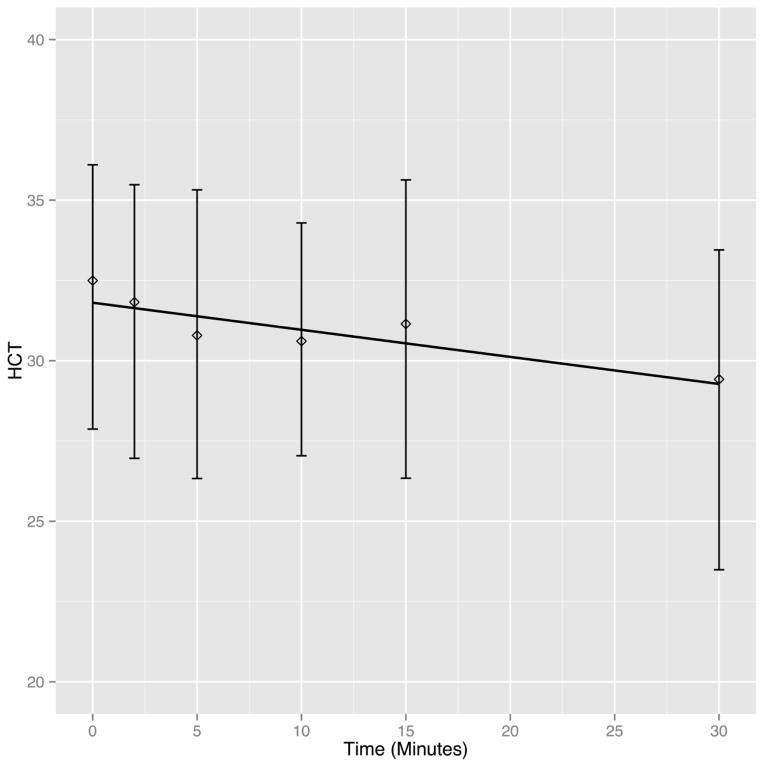
As shown in table 1 and figure 4, there was a statistically significant decrease (p<0.001) in serum hematocrit over histotripsy treatment time (Baseline 32.5 ± 3.6 vs. Post-treatment 29.4 ± 4.2%). Relative to baseline, the expected decrease in serum hematocrit after 30 minutes of histotripsy was 2.52% (Table 1). There was a statistically significant (p<0.001) increase in free hemoglobin (Baseline 6.2 ± 4.6 vs. Post-treatment 348 ± 100 mg/dL) and LDH (Baseline 365 ± 67.8 ± vs. Post-treatment 722 ± 84.7 U/L). The expected rise in free hemoglobin and LDH after histotripsy treatment predicted by the mixed linear effects model was 280.47% and 314.24%, respectively. Additionally, the rate of rise decreased over time for both free hemoglobin (Time2 regression coefficient = −0.25, p<0.001) and LDH (Time2 regression coefficient = −0.12, p<0.001). With regards to hemodynamic variables, there was a statistically significant (p<0.001) increase in RVSP with histotripsy (Baseline 23.2 ± 7.2 vs. Post-treatment 39.7 ± 12 mmHg; expected increase 6.45%), and the rate of rise in RVSP decreased over time (Time2 = −0.06, p<0.001). Apart from this, there were no other clinically significant sequelae of intravascular hemolysis. Specifically, there was no significant (p=0.734) change in heart rate (Baseline 93 ± 18 vs. Post-treatment 92 ± 16 bpm; expected decrease 2.76%) or systemic systolic blood pressure (Baseline 87.9 ± 13 vs. Post-treatment 89.7 ± 18 mmHg; expected decrease 1.38%). There was a small but statistically significant (p<0.001) increase in serum potassium (Baseline 4.2 ± 0.4 vs. Post-treatment 4.8 ± 0.3 mmol/L; expected increase 0.3%), and the rate of rise decreased over time (Time2 regression coefficient = −0.002). Of note, there were no significant electrocardiographic changes consistent with hyperkalemia. Additionally, there was no significant change (p=0.118) in serum creatinine following histotriopsy (Baseline 1.1 ± 0.2 vs. Post-treatment 1.2 ± 0.2 mmol/L; expected increase 0.12%).
Table 1.
Immediate histotripsy effects over 30 minute treatment time
| Time (Minutes)* | Regression Model† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 | 5 | 10 | 15 | 30 | Time‡ | Time2‡ | P‡ | Expected Change Over 30 minutes§ | |
| Hematocrit (%) | 32.5 ± 3.6 | 31.8 ± 3.4 | 30.8 ± 3.8 | 30.6 ± 3.3 | 31.1 ± 3.4 | 29.4 ± 4.2 | −0.084 | - | <0.001 | −2.52 |
| Free Hgb (mg/dl) | 6.2 ± 4.6 | 58.2 ± 15.8 | 102 ± 31.5 | 156 ± 58.4 | 216 ± 86.6 | 348 ± 100 | 16.909 | −0.252 | <0.001 | 280.47 |
| LDH (U/L) | 365 ± 67.8 | 392 ± 77.8 | 431 ± 72.5 | 493 ± 87.1 | 558 ± 102 | 722 ± 84.7 | 13.958 | −0.115 | <0.001 | 314.24 |
| K (mmol/L) | 4.2 ± 0.4 | 4.4 ± 0.5 | 4.6 ± 0.7 | 4.9 ± 0.9 | 5.2 ± 1.2 | 4.8 ± 0.3 | 0.100 | −0.003 | <0.001 | +0.30 |
| WBC (K/μL) | 15.3 ± 3.4 | 11.5 ± 2.7 | 10.6 ± 3.1 | 10.9 ± 3.2 | 11.5 ± 2.7 | 11.9 ± 2.7 | −0.369 | 0.010 | <0.001 | −2.07 |
| Platelet (K/μL) | 398 ± 102 | 318 ± 68.6 | 316 ± 66.1 | 305 ± 107 | 323 ± 82.5 | 344 ± 79.3 | −7.941 | 0.224 | <0.001 | −36.63 |
| Creatinine (mmol/L) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.2 | 0.004 | - | 0.118 | 0.12 |
| RVSP (mmHg) | 23.2 ± 7.2 | 31.9 ± 7.7 | 36.7 ± 11 | 39.3 ± 15 | 42.9 ± 13 | 39.7 ± 12 | 2.105 | −0.063 | <0.001 | 6.45 |
| SBP (mmHg) | 87.9 ± 13 | 96.3 ± 18 | 82.4 ± 28 | 85.8 ± 24 | 99.5 ± 34 | 89.7 ± 18 | −0.046 | - | 0.873 | −1.38 |
| HR (bpm) | 93 ± 18 | 88 ± 14 | 87 ± 22 | 85 ± 20 | 93 ± 39 | 92 ± 16 | −0.092 | - | 0.734 | −2.76 |
Abbreviations: Free Hgb, Serum free hemoglobin; LDH, lactate dehydrogenase; K, serum potassium; WBC, white blood cell count; RVSP, right ventricle systolic blood pressure; SBP, systemic systolic blood pressure; HR, Heart rate; P, likelihood ratio p-value.
Data are presented as mean ± standard deviation.
Linear mixed effects regression model testing the association between treatment time in minutes and change in serum and hemodynamic related analytes
The terms “Time” and “Time2” correspond to the linear and quadratic regression coefficients for time (in minutes) in each model. The “-“ for “Time2” indicates that this squared term was not significant and therefore not estimated in the final model. The likelihood ratio p-value corresponds to the overall (i.e. “Time” alone or “Time” and “Time2” in the quadratic term was significant) association with treatment time.
This is the expected change in a measure over the 30 minute histotripsy treatment time as calculated from the regression model. This value is equal to the value of “Time*30minutes” for those without a significant quadratic term and “Time*30minutes + Time2*30minutes2” for those with a significant quadratic term. For example, the expected change over the 30 minute treatment time for free hemoglobin is 16.909*30 0.252*302=280.47 mg/dL.
Sample size for each time point varied slightly due to death during histotripsy in 4 animals
Figure 4.
Hematocrit declines over histotripsy treatment time. The solid line depicts the linear decrease of hematocrit over time depicted by the linear mixed effects model including both heparin treated and non-treated pigs. The diamond indicates the mean of all hematocrit measurements at each time point among the pigs that survived at that time point, and the vertical bars encompass the 10th to 90th percentile.
Along with mildly decreased hematocrit, there were small, but statistically significant (p<0.001) decreases in white blood cell (WBC) count (Baseline 15.6 ± 3.4 vs. Post-treatment 11.9 ± 2.7 K/μL; expected decrease 2.07%) and platelet count (Baseline 397 ± 102 vs. Post-treatment 344 ± 79 K/μL; expected decrease 36.63%). The rate of fall in the WBC count decreased over time (Time2 regression coefficient = −0.369, p<0.001), as did the rate of fall in the platelet count (Time2 regression coefficient = 0.225, p=0.004).
When comparing heparin and non-heparin treated animals, the two groups were mostly similar with regards to the measured serum and hemodynamic variables. However, heparin treated animals were more likely to have increased RVSP (p=0.036) compared to non-heparin treated animals.
Follow-Up Studies
Of the 17 animals that survived histotripsy, serum laboratory data was available for 16 pigs 48 to 72 hours following histotripsy. All surviving animals were noted to have red tinged urine after returning to the animal housing facility, which subjectively resolved prior to follow up evaluation at 48 to 72 hours. All animals returned to their baseline behavior and fed normally. As seen in Table 2, serum hematocrit remained slightly decreased at follow up when compared to baseline (Baseline 32.7 ± 3.7 vs. Follow-up 29.3 ± 3.8%, p=0.005). LDH also remained elevated (Baseline 363 ± 58 vs. Follow-up 549 ± 88 U/L, p<0.001). Free hemoglobin also remained slightly elevated when compared to baseline (Baseline 6.5 ± 5 vs. Follow-up 8.7 ± 4.3 mg/dL, p<0.001). WBC count, which was decreased immediately following histotripsy, returned to baseline at follow up (Baseline 14.1 ± 3.1 vs. Follow-up 15.9 ± 4.5 K/μL, p=0.804) as did platelet count (Baseline 388 ± 83 vs. Follow-up 374 ± 106 K/μL, p=0.679). Serum potassium was slightly decreased at follow-up (Baseline 4.2 ± 0.3 vs. Follow-up 3.8 ± 0.3 mmol/L, p=0.011). Creatinine remained stable following histotripsy (Baseline 1.1 ± 0.2 vs. Follow-up 1.1 ± 0.2 mmol/L, p=0.932).
Table 2.
Histotripsy Follow Up
| Baseline | 48–72h Follow up | p | |
|---|---|---|---|
| Hematocrit (%) | 32.7 ± 3.7 | 29.3 ± 3.8 | 0.005 |
| Free Hgb (mg/dl) | 6.5 ± 5.0 | 8.7 ± 4.3 | <0.001 |
| LDH (U/L) | 363 ± 58.2 | 549 ± 87.8 | <0.001 |
| K (mmol/L) | 4.2 ± 0.3 | 3.8 ± 0.3 | 0.011 |
| WBC | 14.1 ± 3.1 | 15.9 ± 4.5 | 0.804 |
| Platelet | 388 ± 83 | 374 ± 106 | 0.679 |
| Creatinine | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.932 |
Data are presented as mean ± standard deviation
P values calculated from the paired sample Wilcoxan signed rank test
Discussion
This study demonstrates that histotripsy, targeting free flowing blood, is associated with mild intravascular hemolysis and provides evidence to support systemic anticoagulation during histotripsy therapy. Absence of heparin prior to histotripsy was associated with high mortality albeit under the extreme case scenario of this experiment. This study also adds to the growing body of evidence describing the feasibility and general safety of this technique (2,3,10).
While other forms of therapeutic ultrasound are associated with hemolysis, this had not been previously evaluated with histotripsy. As noted above, our study design maximized adverse effects of histotripsy. Clinical histotripsy applications would be less traumatic to circulating blood, as the primary therapeutic targets in such cases are tissue or thrombus (2–4). Also, the total histotripsy duration in this study (30 minutes) was longer than previously reported treatment times for cardiovascular applications in our laboratory such as thrombolysis (18 minutes), ventricular septal defect creation (4 minutes), or atrial septal defect creation (16 minutes) (2,3,11). We hypothesized that deliberately targeting the femoral vein would cause substantial histotripsy mediated hemolysis by mechanical fractionation in a time dependent fashion. However, despite the extreme case scenario of this experiment, we did not find any clinically significant hemolysis induced anemia. Intravascular hemolysis can also be associated with life threatening hyperkalemia (12). With histotripsy, however, serum potassium was only mildly increased, and there were no electrocardiographic signs of hyperkalemia. Cell free hemoglobin due to hemolysis is an additional concern, as this has been associated with cytotoxic effects on the vascular endothelium as well as acute renal failure (13). With histotripsy, however, there were no signs of acute kidney injury as evidenced by a stably normal creatinine immediately, and 48 to 72 hours, following histotripsy. While we observed transiently increased free hemoglobin during histotripsy, which increased in a quadratic time dependent fashion, the free hemoglobin rise compares favorably to invasive rheolytic intravascular thrombolysis systems, such as the AngioJet (Boston Scientific, Marlborough, MA), and ultrasonic systems, such as OmniWave (Omnisonics, Lakewood, NY). A previous study in pigs found peak free hemoglobin of 1,367 mg/dL after 10 minutes of AngioJet therapy (14). After 10 minutes of OmniWave therapy, peak free hemoglobin was 228 mg/dL (14). After 10 minutes of histotripsy in this study, free hemoglobin was 156 ± 58.4 mg/dL in the surviving animals. While our results compare favorably to other invasive thrombolysis systems, the acute rise in non-compartmentalized hemoglobin may still be clinically important, as free hemoglobin is an avid nitric oxide scavenger (7). Naturally occurring disease states, such as hemoglobinopathies associated with intravascular hemolysis, as well as clinical trials of cell free hemoglobin infusions, have been associated with pulmonary hypertension attributed to nitric oxide scavenging by free hemoglobin (15,16). Our study demonstrated a transient, statistically significant increase in RVSP, which may be due to free hemoglobin mediated nitric oxide scavenging and pulmonary vasoconstriction. The rise in RVSP was significantly greater in the heparin treated group. We hypothesize that this group, in which there was no mortality, had a greater mean treatment time with a normal cardiac output, thereby exposing a larger blood volume to the effects of histotripsy.
Notably, all of the heparin treated animals survived histotripsy, while 46% of non-heparinized animals expired shortly after histotripsy initiation. The survival benefit of heparin may be conferred through multiple modalities. Heparin has been demonstrated to have anti-inflammatory properties and can potentially stabilize endothelial cell function (8,9,17). Heparin has also been shown to activate endothelial nitric oxide synthase (eNOS), leading to vascular relaxation (9). Upregulation of eNOS and increased endothelial nitric oxide production may attenuate the adverse effects associated with free hemoglobin mediated nitric oxide scavenging. Nitric oxide depletion leads to increased platelet activation, adhesion, and aggregation (18). Free hemoglobin can also directly activate platelets (19). Diffuse and systemic platelet activation could potentially mediate sudden death. For example, a murine model of massive intravascular hemolysis resulted in fatal pulmonary arterial hypertension (20). The mechanism of sudden death and pulmonary hypertension in this murine model was attributed to diffuse platelet activation and in situ thrombosis in pulmonary perialveolar vessels (20). Heparin binds to antithrombin III and manifests its primary anticoagulant effect by inhibiting thrombin (21). As thrombin is a potent platelet activator, the indirect effects of heparin on platelet function inhibition may counterbalance the platelet activating effects of free hemoglobin and nitric oxide depletion, thereby decreasing mortality in animals treated with heparin prior to histotripsy (22,23).
There are several limitations to this study. While heparin administration was associated with decreased mortality, the mechanisms conferring heparin protection were not evaluated. In general, pigs are considered hypercoaguable relative to other species (24). If the protective role of heparin is based on its anti-coagulative effect, our findings may overestimate the potential harms of histotripsy without heparin in humans, who may be less likely to form lethal micro-thrombi relative to pigs. Additionally, as noted above, our experimental design established a worst case scenario that maximized hemolysis, and may therefore overestimate the potential harms of histotripsy associated with hemolysis. Clinical applications that target tissue or thrombus, as opposed to free flowing blood may result in less hemolysis. Among animals that died during histotripsy, a complete pathological exam was not performed that may have helped determine the mechanism of death.
In summary, histotripsy targeting free-flowing blood is associated with only mild, transient hemolysis and mildly increased RVSP. Heparin appears to eliminate mortality during histotripsy.
Footnotes
Disclosures
Rajiv Devanagondi: This author has nothing to disclose
Xi Zhang: This author has nothing to disclose
Zhen Xu: Member of the clinical advisory board for Histosonics, Inc. and is named as an inventor on patents filed for applications of histotripsy.
Kimberly Ives: This author has nothing to disclose
Albert Levin: This author has nothing to disclose
Hitinder Gurm: Named as an inventor on a patent filed for applications of histotripsy for thrombolysis by the University of Michigan
Gabe Owens: This author has nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu Z, Ludomirsky A, Eun LY, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–36. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Xu Z. Noninvasive Treatment of Deep Venous Thrombosis Using Pulsed Ultrasound Cavitation Therapy (Histotripsy) in a Porcine Model. Journal of Vascular and Interventional Radiology. 2011;22:369–377. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GE, Miller RM, Ensing G, et al. Therapeutic ultrasound to noninvasively create intracardiac communications in an intact animal model. Catheter Cardiovasc Interv. 2011;77:580–588. doi: 10.1002/ccd.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y, Gelehrter SK, Fifer CG, et al. Non-invasive pulsed cavitational ultrasound for fetal tissue ablation: feasibility study in a fetal sheep model. Ultrasound Obstet Gynecol. 2011;37:450–457. doi: 10.1002/uog.8880. [DOI] [PubMed] [Google Scholar]
- 5.Williams AR, Miller DL, Gross DR. Haemolysis in vivo by therapeutic intensities of ultrasound. Ultrasound Med Biol. 1986;12:501–509. doi: 10.1016/0301-5629(86)90221-8. [DOI] [PubMed] [Google Scholar]
- 6.Poliachik SL, Chandler WL, Mourad PD, et al. Effect of High-Intensity Focused Ultrasound on Whole Blood With and Without Microbubble Contrast Agent. Ultrasound in Medicine & Biology. 1999;25:991–998. doi: 10.1016/s0301-5629(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 7.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 8.Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 1990;171:2025–2041. doi: 10.1084/jem.171.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouretas PC, Hannan RL, Kapur NK, et al. Non-anticoagulant heparin increases endothelial nitric oxide synthase activity: role of inhibitory guanine nucleotide proteins. J Mol Cell Cardiol. 1998;30:2669–2682. doi: 10.1006/jmcc.1998.0831. [DOI] [PubMed] [Google Scholar]
- 10.Owens GE, Miller RM, Owens ST, et al. Intermediate-Term Effects of Intracardiac Communications Created Noninvasively by Therapeutic Ultrasound (Histotripsy) in a Porcine Model. Pediatric Cardiology. 2011;33:83–89. doi: 10.1007/s00246-011-0094-6. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121:742–749. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Soriano J. Potassium homeostasis and its disturbances in children. Pediatr Nephrol. 1995;9:364–374. doi: 10.1007/BF02254217. [DOI] [PubMed] [Google Scholar]
- 13.Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 14.Lang EV, Kulis AM, Villani M, Barnhart W, Balano R, Cohen R. Hemolysis comparison between the OmniSonics OmniWave Endovascular System and the Possis AngioJet in a porcine model. J Vasc Interv Radiol. 2008;19:1215–1221. doi: 10.1016/j.jvir.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 16.Lamy ML, Daily EK, Brichant JF, et al. Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. The DCLHb Cardiac Surgery Trial Collaborative Group. Anesthesiology. 2000;92:646–656. doi: 10.1097/00000542-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lever R, Page CP. Non-anticoagulant effects of heparin: an overview. Handb Exp Pharmacol. 2012;207:281–305. doi: 10.1007/978-3-642-23056-1_12. [DOI] [PubMed] [Google Scholar]
- 18.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 19.Iuliano L, Violi F, Pedersen JZ, Pratico D, Rotilio G, Balsano F. Free radical-mediated platelet activation by hemoglobin released from red blood cells. Arch Biochem Biophys. 1992;299:220–224. doi: 10.1016/0003-9861(92)90267-z. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Jin R, Zhang J, et al. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116:1613–1622. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsh J, Dalen JE, Deykin D, Poller L. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1992;102:337S–351S. doi: 10.1378/chest.102.4_supplement.337s. [DOI] [PubMed] [Google Scholar]
- 22.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 23.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 24.Palsgaard-Van Lue A, Strom H, Lee MH, et al. Cellular, hemostatic, and inflammatory paramaeters of the surgical stress response in pigs undergoing partial pericardectomy via open thoracotomy or thorascopy. Surg Endosc. 2007;21:785–792. doi: 10.1007/s00464-006-9033-7. [DOI] [PubMed] [Google Scholar]