Abstract
Rett syndrome is a Pervasive Developmental Disorder (PDD) associated with de novo mutations of the methyl CpG-binding protein 2 (MECP2) gene. Mecp2 functions as a transcription factor that regulating the expression of hundreds of genes. Identification of the role of Mecp2 in specific neurodevelopmental symptoms remains an important research aim. We previously demonstrated that male mice possessing a truncation mutation in Mecp2 are hyper-social. We predicted that reduced fear or anxiety might underlie this enhanced affiliation. In order to probe risk assessment and anxiety-like behavior, we compared Mecp2 truncation mutants to their wild-type littermates in the elevated plus maze and elevated zero maze. Additionally, subjects were administered the mouse defense test battery to evaluate unconditioned fear- and panic-like behavior to a graded set of threat scenarios and a predator stimulus. Mutant mice showed no significant changes in anxiety-like behavior. Yet, they displayed hyper-reactive escape and defensive behaviors to an animate predatory threat stimulus. Notably, mutant mice engaged in exaggerated active defense responding to threat stimuli at nearly all phases of the fear battery. These results reveal abnormalities in emotion regulation in Mecp2 mutants particularly in response to ecologically relevant threats. This hyper-responsivity suggests that transcriptional targets of Mecp2 are critical to emotion regulation. Moreover, we suggest that detailed analysis of defensive behavior and aggression with ethologically relevant tasks provides an avenue to interrogate gene-behavior mechanisms neurodevelopmental and other psychiatric conditions.
Keywords: MECP2, Rett Syndrome, Ethoexperimental Approach, Autism, Defense, Fear
1.0 Introduction
Mouse models of neurodevelopmental disorders have displayed outstanding face validity and facilitate widespread investigation into the biology of Pervasive Developmental Disorder (PDD) [1]. Yet, most tasks have emphasized high throughput in order to support extensive genetic and pharmacological discovery designs. The Blanchard laboratory at the University of Hawai'i at Mānoa, under the leadership of the late Dr. Robert Blanchard has a rich history of developing rodent behavioral tasks deriving from an unparalleled expertise in behavior function and ecological context. The lab's ethoexperimental approach makes careful use of tasks and arenas approximating scenarios encountered in the wild but with the added benefit of controlled experimental conditions [2]. Application of the ethoexperimental approach to genetic mouse models of Autism Spectrum Disorder (ASD) has been particularly beneficial since ASD is characterized by social impairments and emotional dysfunction. To garner insight into the social and emotional phenotype of mouse models of ASD, the Blanchard lab has systematically investigated inbred mouse strains [3-6] and mutant models of ASD [7,8] using in-depth investigation into the quality and form of social interactions as well as restricted, repetitive behaviors. All strains and mutants were assessed in the visible burrow system (VBS), a colony-housing context that recapitulates natural life history characteristics of mice and permits long-term monitoring of complex social behaviors. While many models displayed social impairment in the VBS (avoidance, elevated agonism, etc) MeCP2308/y mice preferred social contact and made more frontal approaches than colonies of congenic, wild-type littermates [8]. They also made more affiliative investigations of a stimulus mouse than did their wild-type littermates and displayed a virtual absence of home-cage territorial aggression. This led to the hypothesis that Mecp2 mutants may possess dysregulated emotion systems rendering them less averse to potentially threatening stimuli. Therefore, we predicted that Mecp2 mutant mice would be less anxious and fearful in a battery of emotion-relevant behavioral tasks as evidenced by reductions in flight, freezing and defensive behaviors.
Methyl CpG binding protein 2 (Mecp2) is a DNA binding protein intricately involved in the transcription of a variety of genes [9-14]. Mecp2 recognizes methylated DNA in gene promoter regions and recruits transcriptional modulators such as histone deacetylases that alter chromatin packaging [15]. Not only does MECP2 mutation underlie most cases of Rett syndrome [16,17], the gene and its locus are implicated in non-syndromic autism [18-21] and other psychiatric disorders [22,23]. Rett syndrome (ICD-10-CM: F84.2) is an X-linked neurodevelopmental disorder identified almost exclusively in females due to lethality in males lacking a second X chromosome. It is also characterized by a period of seemingly normal development and functioning followed by a period of rapid regression where most purposeful hand movements and language are lost [24]. Mice with Mecp2 mutations show symptoms of pervasive neurodevelopmental disorders [25,26]. In addition to sleep, breathing and musculoskeletal abnormalities resembling those of Rett syndrome, many Mecp2 mutant mice display aberrant behaviors characteristic of cognitive and emotional disturbances [27-38]. Thus, the quantity, composition, and distribution of Mecp2 in early brain development and in the adult [39,40] is critical for normal brain functioning [41]. Indeed, mutation of MECP2 causes severe and widespread alterations in brain cellular and molecular physiology [38,42-46]. Post-mortem tissue from Rett syndrome- diagnosed donors as well as those collected from Mecp2 mutant mice exhibit severe and diffuse changes in brain cell and synaptic characteristics [47-52].
Relative to the somatic manifestations of the condition the proximate mechanisms causing affective and emotional functions in Rett syndrome [53] are perhaps less understood. Rett syndrome is associated with elevated anxiety [53,54] and panic-like behavior [55]. Since individuals diagnosed with Rett syndrome are largely non-verbal [56] this severely limits the ability to probe the psychological well being of an individual with the condition through self-report [57,58]. Therefore, careful investigation of emotion systems in animal models can help identify of brain circuit alterations that manifest in adverse psychological symptoms. With careful attention to behavioral function and form we careful interrogated the emotional repertoire of the MeCP2308/y mutant model of Rett syndrome.
2.0 Materials and Methods
2.1 Experimental Subjects
MeCP2308/Y mice were bred from C57BL/6J-backcrossed stock obtained from Jackson Laboratory (B6.129S-Mecp2tm1Hzo/J, stock #005439) originally derived from the truncation mutation described in Shahbazian and colleagues [36]. Subjects were littermates bred from heterozygous mutant dams and hemizygous father sibling pairs. Subject mouse genotype was determined according to the PCR parameters from The Jackson Laboratory. Wild type (Y/+: WT) and hemizygous (Y/-: KO) male mice, aged 8-13 weeks were used for experimentation. Mice were housed with up to five same-sex littermates under a 12-h light/dark schedule with lights on at 06:00h. Male mice were tested to reduce variability associated with mosaicism in Mecp2 truncation in expression in female mice. Sprague Dawley rats were bred in house to be used as a predatory stimulus. Rodent subjects had ad libitum access to tap water and lab rodent diet. All procedures were performed according to protocols approved by the University of Hawaii Laboratory Animal Service Institutional Animal Care and Use Committee.
2.2 Behavioral Testing
2.2.1 Elevated Plus Maze (EPM)
The test apparatus is based on that described by Lister [59]. It is composed of two open arms (30 × 7 × 2 cm) and two closed arms (30 × 7 × 20 cm) that extend from a common central platform (5 × 5 cm). The apparatus was constructed from wood and Plexiglas and was raised to a height of 40 cm above floor level. To prevent mice falling off, a rim of Plexiglas (0.25 cm high) surrounded the perimeter of the open arms. One ceiling-mounted video camera was used to record mouse behavior during the test and the experimental room was illuminated with standard fluorescent lamps. The mean intensity of luminosity on the open arms of the EPM was 55 lux. Testing was initiated by placing the subject on the central platform of the maze, facing one of the open arms. Test sessions lasted five minutes and, between subjects, the maze was thoroughly cleaned with 70% ethanol and dried with paper towels. The results were expressed as frequency of entries into the closed and open arms, as well as percent time spent in open arms with respect to total time in both closed and open arms. Risk assessment measures included frequency scores for stretch attend postures where the mouse stretched forward and maintained a “flat-back” posture, and frequency of head dips where the mouse protruded its head over the edge of an open arm to a length greater than or exceeding the caudal aspect of the pinnae.
2.2.2 Elevated Zero Maze (EZM)
The test apparatus consisted of an acrylic circular platform (6 cm in width) composed of open and closed segments, with the latter being surrounded by 20 cm high acrylic walls. The platform was raised to a height of 50 cm above floor level and the diameter of the maze was 65 cm. To prevent mice falling off, a rim of Plexiglas (0.25 cm high) surrounded the perimeter of the open segments. One ceiling-mounted video camera was used to record behavior during the test and the experimental room was illuminated with standard fluorescent lamps. The mean intensity of luminosity on the open segments of the zero-maze was 70 lux. Testing was initiated by placing the subject in the center of the open arm. Test sessions lasted 5 minutes. The results were expressed as mean entries into the closed and open sections as well as mean percentage of time spent in open segments over total time spent in both open and closed segments. Risk assessment measures included frequency scores for stretch attend postures and head dips. Videotapes collected for the elevated plus and elevated zero mazes were scored by an observer blind to genotype using commercially available annotation software (Observer, Noldus Information Technology, The Netherlands). Eight to nine mice per genotype naïve to experimental experience were tested in the EPM and EZM. Entries and percent time in a particular zone of the plus or zero maze required that the mouse place all four paws cross the threshold.
2.2.3 Mouse Defense Test Battery (MDTB)
Nine mice of each genotype previously tested in the EZM were tested in the MDTB after waiting at least one week to prevent stress from repeated testing. The mouse defense test battery was performed according to published protocols [60]. An oval arena measuring 40 cm wide, 30 cm high and 480 cm in length, consisting of two 200 cm straight segments joined by two 40 cm curved segments and separated by a median wall (200 cm long x 30 cm high). The black Plexiglas arena was elevated 80 cm from the floor to facilitate predatory stimulus exposure and to block the subject's view of the experimenter. Lighting intensity was controlled by a single red incandescent light bulb producing 1 lux on the floor of the arena and 5 lux at the top. The floor of the apparatus was marked every 20 cm with white lines to facilitate measurement of locomotion distances and velocity. Two ceiling-mounted video cameras were used to record behaviors during the test. Researchers performing the MDTB were blind to subject genotype during the test and during manual offline scoring of line crossings and rearing behavior. All other behaviors were scored in real time. An adult Sprague-Dawley rat (average weight of 450 g) was deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and utilized as a threatening predatory stimulus. The MDTB consisted of the following subtests:
Pre-test
Subjects were placed into the MDTB apparatus for a 180 second familiarization period during which total line crossings and wall rears were recorded.
Predator avoidance test
A predator stimulus (a hand-held rat) was brought up to the subject at a speed of approximately 0.5 m/second. Approach was terminated when contact with the subject was made or if the subject ran away from the approaching rat. The distance at which the mice turned to avoid the predator and the escape distance (the length the mouse traveled after rat movement was terminated) were noted. This was repeated five times.
Chase/flight test
The hand-held rat was brought up to the subject at a speed of approximately 2.0 m/second. Chase was initiated only when the subject was standing still with its head oriented toward the rat, and completed when the subject had traveled three laps in the runway (14.4 m). The time spent by the mouse to travel this distance was recorded. The number of stops (pauses in locomotion), reversals (subject turned and ran toward the rat), and jump escapes (subject jumps to avoid the rat stimulus) were also recorded. The latency to complete the total distance was used to calculate mean flight speed in meters per second (m/second).
Closed Door Approach Test
The runway was then converted into a straight alley, 80 cm long, by the closing of a door at one end and the placement of a removable barrier at the other. The rat was placed at one end and during a 30 second period, the number of approach-withdrawals (subject moves toward the rat more than 20 cm and then returns), as well as voluntary contacts with the rat stimulus were recorded. During this phase, the investigator rotated the rat from side-to-side slowly to act as an animate but stationary predatory. Other measures scored included the frequency of defensive uprights and immobility time (freezing).
Forced contact test
The alley was reduced to 40 cm. The experimenter applied the anesthetized rat to the mouse in five sudden contacts. For each such contact, the number of vocalizations, defensive uprights, jumps escapes, jump attacks and bites were recorded. This procedure was repeated three times.
Post-test
Upon completion of the forced contact test, the alley doors were opened and the subject's line crossings and wall rears were record as an index of locomotion and exploration.
2.3 Statistical Analyses
Mean values of parameters in each phase of the elevated mazes were compared with two-way repeated measures analysis of variance (ANOVA) with Bonferroni post-hoc analyses conducted when significant main effect for genotype was identified. Results for the mouse defense test battery were compared with unpaired t-tests. Mann-Whitney U-tests were substituted where assumptions were violated such as in cases of unequal variance.
3.0 Results
Mice displayed no obvious motor or respiratory impairments during testing nor was there any mortality.
3.1 EPM
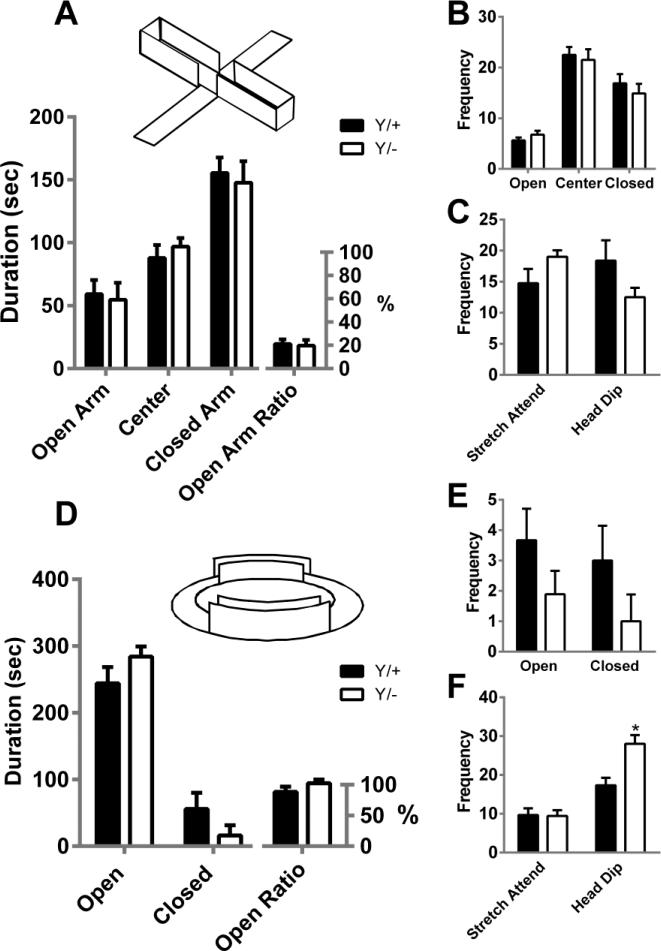
Figure 1 displays parameters collected in the EPM and EZM. In the EPM, no significant genotype differences were identified in the duration of time spent in the open, center and closed arms of the elevated plus maze nor in the proportion of time spent in the open arms relative to the closed sections (F1,42=0.51, p=0.49; Fig, 1A). No significant genotype differences were noted in the frequency of entries into the arms of the EPM (F1,28=0.12, p=0.73; Fig. 1B) nor in the frequency of stretch attend postures and exploratory head dips (F1,14=0.68, p=0.68; Fig. 1C).
Figure 1. Anxiety-like behavior.
Mutant wild-type (Y/+) and MeCP2308/y (Y/−) mice exhibit similar amounts of time spent in all areas of the elevated plus-maze (A). No significant differences in mean frequencies of entries into the three zones (B), or in the frequencies of stretch attend or head dip behaviors (C) were found for the two genotypes. In the elevated zero-maze, no significant differences were found in the mean duration of time spent in the open or closed compartments (D) and although mutant mice appeared to show lower total entries into the compartments (E), this effect was not statistically significant. Mutant mice displayed comparable frequencies of stretch attend risk assessment behaviors while performing more head dips in the elevated zero-maze (F). *p<0.001. n=8-9/genotype.
3.2 EZM
In the EZM, mutant mice showed no significant differences in the duration of time spent in the open or closed runways, nor the proportion of time in the open compartment (F1,32=1.90, p=0.19; Fig. 1D). Entry frequencies were also similar between genotypes (F1,16=1.91, p=0.19; Fig. 1E). There was an overall genotype difference in risk assessment in the elevated zero-maze (F1,16=8.11, p=0.01; Fig. 1F). Although the two genotypes displayed similar rates of stretch attend postures, post-hoc comparison indicated that mutant mice showed a significant elevation in the frequency of head dips (p<0.001).
3.3 MDTB
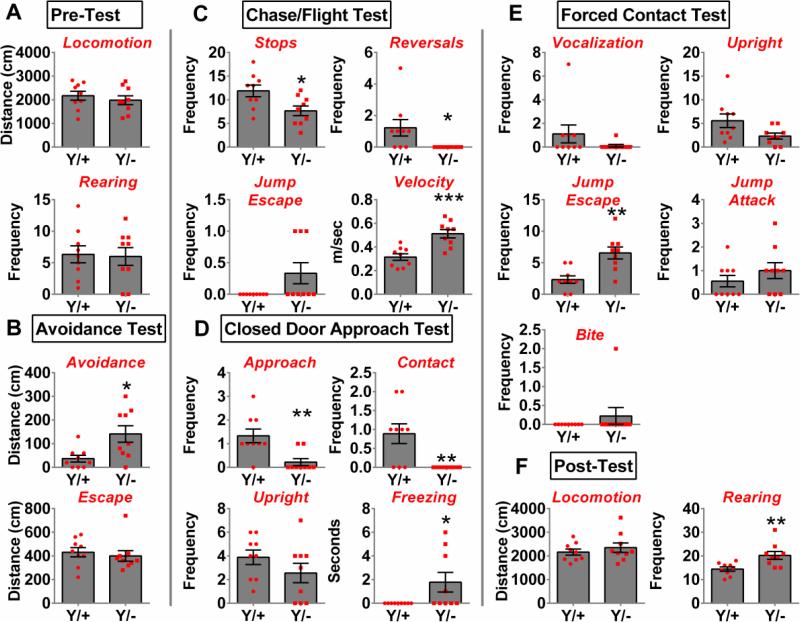
Figure 2 displays the mean and standard error for MDTB parameters. Genotypes did not differ in locomotion (p=0.49) or exploratory rearing (p=0.87) in the pre-test (Fig 2A). When initially exposed to the predator, Mecp2 mutants showed an enhanced avoidance distance (Mann Whitney, p=0.02), but a similar escape distance (p=0.61) relative to wild-type controls (Fig 2B). Mutants showed significantly fewer stops (p=0.02) and reversals (Mann Whitney, p=0.01) and enhanced flight velocity (p<0.001) in the chase/flight component with no significant (Mann Whitney p=0.21) increase in jump escape behavior (Fig 2C). When escape was blocked in the closed door approach test, mutants performed fewer approaches (p<0.01) and contacts (Mann Whitney p<0.01) and instead displayed a similar (Mann Whitney p=0.08) duration of freezing with no change (p=0.21) in upright exploratory behavior (Fig 2D). When mice were exposed to forced contact with the rat, there was no difference in the frequency of vocalizations (Mann Whitney p=0.24) or defensive upright postures (Mann Whitney p=0.06); however, mutants performed more jump escape behaviors (p<0.01) than wild type mice while displaying similar levels of jump attack (p=0.30) and bite (Mann Whitney p=0.99) responses (Fig 2E). Note however that one Mecp2 mutant engaged in high levels of defensive aggression by biting the rat twice. Once the predatory stimulus was removed (post-test) mutants performed more rearing (p<0.01), an exploratory risk-assessment behavior, while maintaining locomotor activity comparable to wild-type sibling controls (p=0.44). Thus, Mecp2 truncation causes enhanced reactivity to an animate predatory threat stimulus, principally manifesting as an exaggerated avoidance response.
Figure 2. Mouse defense test battery.
In various phases of the escalating predatory threat scenario, mutant mice (Y/−) displayed increased defensive behavior relative to wild-type siblings (Y/+). Mutant mice avoided the predator from a greater distance (B). When chased by the predator, they made fewer stops and never reversed their course, and they showed higher escape velocity (C). When escape was blocked and the predator was located at the opposing end of the arena, the mutants approached less and made fewer contacts with the rat and instead engaged in more freezing (D). Upon forced contact by the rat, mutants engaged in more jump escapes (E). After the stimulus was removed, the mutant mice showed more rearing behaviors (F). *p<0.05, **p<0.01, ***p<0.001. n=9/genotype.
4.0 Discussion
Numerous genes and neural circuits have been implicated in ASD and Rett syndrome, yet, despite this substantial attention, there are no treatments or cures for these highly debilitating neurodevelopmental disorders. Here, we interrogated anxiety- and fear-like behavior in Mecp2 mutant mice. We posited that reliable understanding of a disease mutation on negative emotional behavior requires the use of species-typical defensive responses reliably tied to emotional processes [61]. In standard assays of anxiety-like behavior, Mecp2 mutant mice displayed no difference in anxiety-like behaviors relative to their wild type siblings. Results of studies measuring anxiety in Mecp2 mutants are mixed (see [62] for a review). Our reports are contrary to others demonstrating elevated anxiety in the MeCP2308/Y mutant [28,32,36]. Therefore, it is difficult to reconcile the specific effect of Mecp2 loss of function on anxiety. One could argue that since anxiolytic/anxiogenic compounds validate these paradigms their validity derives from their pharmacological validity. That is to say, they are quite good at identifying chemicals that modulate emotion, but could be considered insensitive to identifying ecologically relevant states of the experimental subject. Additionally, such arena-based tasks require that there are no musculoskeletal, locomotor or proprioceptive impairments in the model. Since MeCP2308/y show altered motor activity, coordination and tremors, it limits the interpretation of some behavioral data [28,36]. Genetic background also appears to play a critical role in the social phenotypes of Mecp2 mutations [63] so perhaps the presence and direction of social and anxiety phenotypes may rely on genetic backgrounds. Our results do not endorse that this mutant (on a fully backcrossed C57Bl/6J background) exhibits robust anxiety-like changes in the classic elevated maze paradigms.
To the best of our knowledge, unconditioned fear-like responses to a predator have not been evaluated in a Mecp2 mutant model. We found that in the mouse defense test battery, Mecp2 mutants displayed increased fear- and panic-like behavior. Enhancement in active flight and defensive aggression responses in this task has been shown to reflect panic-like responses amenable to panicolytic compounds, but not anxiolytic ones [64]. These results suggest that Mecp2 loss of function causes specific dysregulation of neural processes central to emotion control. More specifically, this particular mutation seems to result in a fear- and panic-like phenotype not previously identified in nonhuman models of Rett syndrome. Previous work has demonstrated that MeCP2308/Y mutant mice exhibit normal fear conditioning in a context conditioning design but not in a cue-conditioning paradigm [30,36]. However, the MDTB is designed to test unconditioned fear responses and it is worth noting that fear conditioning and unconditioned fear are characterized by unique neurocircuitry [65]. The pattern of responses identified in the current study suggests Mecp2 mutants might be hypersensitive to animate, but not inanimate threat stimuli. This does not appear to extend to conspecifics as this mutant exhibits an absence of territorial defense behavior and enhanced social seeking [8].
4.1 Neuropeptide and endogenous opiate systems are implicated in Mecp2 emotion disturbance
Mecp2 acts as a transcription factor affecting thousands of genes. Mecp2 binds the CpG-island rich promoter of the corticotrophin releasing hormone (Crh) gene regulating its transcription [32]. Given the role of Crh neuropeptide in anxiety and stress it seems a plausible contributor to the emotional alterations in Rett syndrome models. AAV vector based cre recombinase expression in the basolateral amygdala of floxed Mecp2 conditional mice caused increased anxiety-like behavior suggesting involvement of the amydgala [66]. No alteration in Crh mRNA levels were detected in the mice with conditional deletion of Mecp2 in the amygdala suggesting other transcriptional targets could play a significant role in the myriad emotional effects in Mecp2 mutants. A more recent report confirms that Crh and the opioid receptor Oprm1 are Mecp2 targets that modulate the influence of Mecp2 duplication [67]. The specific role of Mecp2 in defensive behavior is supported with research demonstrating risk assessment behaviors correspond to varying Mecp2 levels in mosaic Mecp2+/- female mice [68]. To establish whether known Mecp2 targets underlie the exaggerated defensive behaviors demonstrated here, future experiments could evaluate the behavioral effect of RNA interference or antagonists of Crh receptors or Oprm1 in the MDTB. Evidence for this notion comes from a study establishing that that anxiety-induced breathing abnormalities in Mecp2−/y male mice stem are Crh dependent as the Crh antagonist antalarmin attenuates the response [69]. Notably one of the stimuli used was an odorant (trimethylthiazoline) thought to mimic predatory olfactory stimulation. Antalarmin improved survivability in these null mutants indicating the neuropeptide is a critical Mecp2 target and fear/panic-like behaviors are sensitive indicators of Mecp2 dosage.
4.2 Summary
In the current study we attempted to evaluate whether fear and anxiety are reduced in the MeCP2308/y model of Rett syndrome. Out intention was to place the mutant's hyper-social characteristics into context. Contrary to the prediction that they would have reduced fear and anxiety we identified a pronounced enhancement of defensive responses in multiple phases of the MDTB. We suggest that the ethoexperimental approach can be advantageous in modeling symptoms of complex neuropsychiatric disorders. Comparing the more subjective emotional experience of Rett syndrome patients is limited by their limited ability to communicate [58]. Nonspecific influences of dementia, epilepsy and intellectual deficiency further complicate interpretation of causative and consequential symptomatology. However, some early reports suggest abnormal defensive responses in females with Rett syndrome [56,70]. In fact, many children diagnosed with PDD exhibit comorbid fear (phobias) and anxiety [71]. Alongside attempts to reduce seizures and improve respiratory and musculoskeletal problems in Rett syndrome, improved understanding and treatment of comorbid affective and emotional issues is critical. The current work has established the role of Mecp2 in innate fear-like behavioral responses and could help guide research designed to improve the psychological experiences of persons diagnosed with Rett syndrome.
Acknowledgements
Funded by NIH MH081845 to R.J.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci. 2012;14:293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- 3.Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–51. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson BL, Pobbe RLH, Defensor EB, Oasey L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+ tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Defensor EB, Pearson BL, Pobbe RLH, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav. Brain Res. 2011;217:302–8. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pobbe RLH, Pearson BL, Defensor EB, Bolivar VJ, Young WS, Blanchard DC, Blanchard RJ. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav. 2012;61:436–44. doi: 10.1016/j.yhbeh.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson BL, Defensor EB, Pobbe RLH, Yamamoto LHL, Bolivar VJ, Blanchard DC, Blanchard RJ. MeCP2 truncation in male mice promotes affiliative social behavior. Behav Genet. 2012;42:299–312. doi: 10.1007/s10519-011-9501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–9. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen HF, Bird A. MeCP2 and other methyl-CpG binding proteins. Ment Retard Dev Disabil Res Rev. 2002;8:87–93. doi: 10.1002/mrdd.10021. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–14. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 12.Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, Greer JJ, Percy A, Glaze DG, Zoghbi HY, Neul JL. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci USA. 2009;106:21966–71. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Tao J, Chen PJ, Shahab A, Ge W, Hart RP, Ruan X, Ruan Y, Sun YE. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2010;107:18161–6. doi: 10.1073/pnas.1005595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, Kang D, Richman R, Johnson JM, Berget S, Zoghbi HY. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci USA. 2005;102:17551–8. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–9. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 16.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 17.Bienvenu T, Carrié A, de Roux N, Vinet MC, Jonveaux P, Couvert P, Villard L, Arzimanoglou A, Beldjord C, Fontes M, Tardieu M, Chelly J. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet. 2000;9:1377–84. doi: 10.1093/hmg/9.9.1377. [DOI] [PubMed] [Google Scholar]
- 18.Beyer KS, Blasi F, Bacchelli E, Klauck SM, Maestrini E, Poustka A. Mutation analysis of the coding sequence of the MECP2 gene in infantile autism. Hum Genet. 2002;111:305–309. doi: 10.1007/s00439-002-0786-3. [DOI] [PubMed] [Google Scholar]
- 19.Carney RM, Wolpert CM, Ravan SA, Shahbazian M, Ashley-Koch A, Cuccaro ML, Vance JM, Pericak-Vance MA. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28:205–11. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 20.Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samaco RC, Nagarajan RP, Braunschweig D, LaSalle JM. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum Mol Genet. 2004;13:629–39. doi: 10.1093/hmg/ddh063. [DOI] [PubMed] [Google Scholar]
- 22.Hammer S, Dorrani N, Dragich J, Kudo S, Schanen C. The phenotypic consequences of MECP2 mutations extend beyond Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:94–8. doi: 10.1002/mrdd.10023. [DOI] [PubMed] [Google Scholar]
- 23.Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, Lugtenberg D, Bienvenu T, Jensen LR, Gecz J, Moraine C, Marynen P, Fryns JP, Froyen G. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77:442–53. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagberg B. Clinical manifestations and stages of rett syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:61–5. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- 25.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–31. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 26.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–6. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 27.Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich J, Aunis D, Zwiller J. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–92. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- 28.De Filippis B, Ricceri L, Laviola G. Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav. 2010;9:213–23. doi: 10.1111/j.1601-183X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 29.Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010;13:1128–36. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–76. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–7. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2006;103:18267–72. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–20. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 34.Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Amstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–27. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PP. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–98. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 36.Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–54. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 37.Stearns NA, Schaevitz LR, Bowling H, Nag N, Berger UV, Berger-Sweeney J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience. 2007;146:907–21. doi: 10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Su D, Cha YM, West AE. Mutation of MeCP2 alters transcriptional regulation of select immediate-early genes. Epigenetics. 2012;7:146–54. doi: 10.4161/epi.7.2.18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGraw CM, Samaco RC, Zoghbi HY. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen MV, Du F, Felice CA, Shan X, Nigam A, Mandel G, Robinson JK, Ballas N. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J Neurosci. 2012;32:10021–34. doi: 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufmann WE, Johnston MV, Blue ME. MeCP2 expression and function during brain development: implications for Rett syndrome's pathogenesis and clinical evolution. Brain Dev. 2005;27:S77–87. doi: 10.1016/j.braindev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–27. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Gantz SC, Ford CP, Neve KA, Williams JT. Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. J Neurosci. 2011;31:12629–37. doi: 10.1523/JNEUROSCI.0684-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishi N, Macklis JD. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp Neurol. 2010;222:51–8. doi: 10.1016/j.expneurol.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roux JC, Zala D, Panayotis N, Borges-Correia A, Saudou F, Villard L. Modification of the Mecp2 dosage alters axonal transport through the Huntingtin/Hap1 pathway. Neurobiol Dis. 2012;45:786–95. doi: 10.1016/j.nbd.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Stuss DP, Boyd JD, Levin DB, Delaney KR. MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS One. 2012;7:e31896. doi: 10.1371/journal.pone.0031896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauman ML, Kemper TL, Arin DM. Microscopic observations of the brain in Rett syndrome. Neuropediatrics. 1995;26:105–8. doi: 10.1055/s-2007-979737. [DOI] [PubMed] [Google Scholar]
- 48.Belichenko PV, Wright EE, Belichenko NP, Masliah E, Li HH, Mobley WC, Francke U. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514:240–58. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- 49.Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis. 2009;35:219–33. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett Syndrome. Proc Natl Acad Sci USA. 2005;102:12560–5. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farra N, Zhang WB, Pasceri P, Eubanks JH, Salter MW, Ellis J. Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations. Mol Psychiatry. 2012;17:1261–71. doi: 10.1038/mp.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto Y. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J Neuropathol Exp Neurol. 2005;64:537–44. doi: 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- 53.Mount RH, Charman T, Hastings RP, Reilly S, Cass H. The Rett Syndrome Behavior Questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. J Child Psychol Psychiatry. 2002;43:1099–110. doi: 10.1111/1469-7610.00236. [DOI] [PubMed] [Google Scholar]
- 54.Sansom D, Krishnan VH, Corbett J, Kerr A. Emotional and behavioural aspects of Rett syndrome. Dev Med Child Neurol. 1993;35:340–45. doi: 10.1111/j.1469-8749.1993.tb11646.x. [DOI] [PubMed] [Google Scholar]
- 55.Christodoulou J, Ho G. MECP2-Related Disorders. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Source: GeneReviews® [Internet] University of Washington, Seattle; Seattle (WA): 2001. [Google Scholar]
- 56.Olsson B, Rett A. Behavioral observations concerning differential diagnosis between the Rett syndrome and autism. Brain Dev. 1985;7:281–9. doi: 10.1016/s0387-7604(85)80029-2. [DOI] [PubMed] [Google Scholar]
- 57.Ozonoff S, Goodlin-Jones BL, Solomon M. Evidence-based assessment of autism spectrum disorders in children and adolescents. J Clin Child Adolesc Psychol. 2005;34:523–40. doi: 10.1207/s15374424jccp3403_8. [DOI] [PubMed] [Google Scholar]
- 58.Quest KM, Byiers BJ, Payen A, Symons FJ. Rett syndrome: a preliminary analysis of stereotypy, stress, and negative affect. Res Dev Disabil. 2014;35:1191–7. doi: 10.1016/j.ridd.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 60.Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- 61.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–18. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 62.Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav Pharmacol. 2008;19:501–17. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- 63.Tantra M, Hammer C, Kästner A, Dahm L, Begemann M, Bodda C, Hammerschmidt K, Giegling I, Stepniak B, Castillo Venzor A, Konte B, Erbaba B, Hartmann A, Tarami A, Schulz-Schaeffer W, Rujescu D, Mannan AU, Ehrenreich H. Mild expression differences of MECP2 influencing aggressive social behavior. EMBO Mol Med. 2014;6:662–84. doi: 10.1002/emmm.201303744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanchard RJ, Griebel G, Henrie JA, Blanchard DC. Differentiation of anxiolytic and panicolytic drugs by effects on rat and mouse defense test batteries. Neurosci Biobehav Rev. 1997;21:783–9. doi: 10.1016/s0149-7634(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 65.Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- 66.Adachi M, Autry AE, Covington HE, 3rd, Monteggia LM. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci. 2009;29:4218–27. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samaco RC, Mandel-Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat Genet 2012. 2012;44:206–11. doi: 10.1038/ng.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wither RG, Lang M, Zhang L, Eubanks JH. Regional MeCP2 expression levels in the female MeCP2-deficient mouse brain correlate with specific behavioral impairments. Exp Neurol. 2013;239:49–59. doi: 10.1016/j.expneurol.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Ren J, Ding X, Funk GD, Greer JJ. Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome. J Neurosci. 2012;32:17230–40. doi: 10.1523/JNEUROSCI.2951-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olsson B, Rett A. Autism and Rett syndrome: behavioural investigations and differential diagnosis. Dev Med Child Neurol. 1987;29:429–41. doi: 10.1111/j.1469-8749.1987.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 71.Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C. Comorbid anxiety symptoms in children with pervasive developmental disorders. J Anxiety Disord. 1998;12:387–93. doi: 10.1016/s0887-6185(98)00022-x. [DOI] [PubMed] [Google Scholar]