Fig. 3.
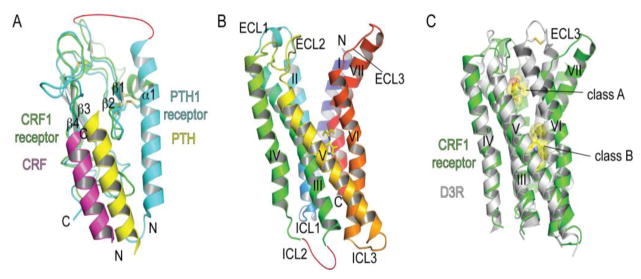
Structures of class B GPCR domains and positions of ligand binding surfaces and pockets. (A) N-terminal extracellular domain structures of the parathyroid hormone PTH1 receptor and corticotropin releasing factor CRF1 receptor with bound PTH (PDB: 3C4M) and CRF (PDB: 3EHU) peptide fragments, respectively. (B) The CRF1 receptor 7TM structure with a bound small molecule antagonist CP-376395 (PDB: 4K5Y) shown in stick representation. (C) Superposition of the class B CRF1 receptor 7TM and class A dopamine D3 receptor with a bound antagonist eticlopride (PDB: 3PBL) highlighting the difference in positions of the antagonist drug binding pockets and the open cleft in the CRF1 receptor. Ligands are shown in stick and space-filling representation.
