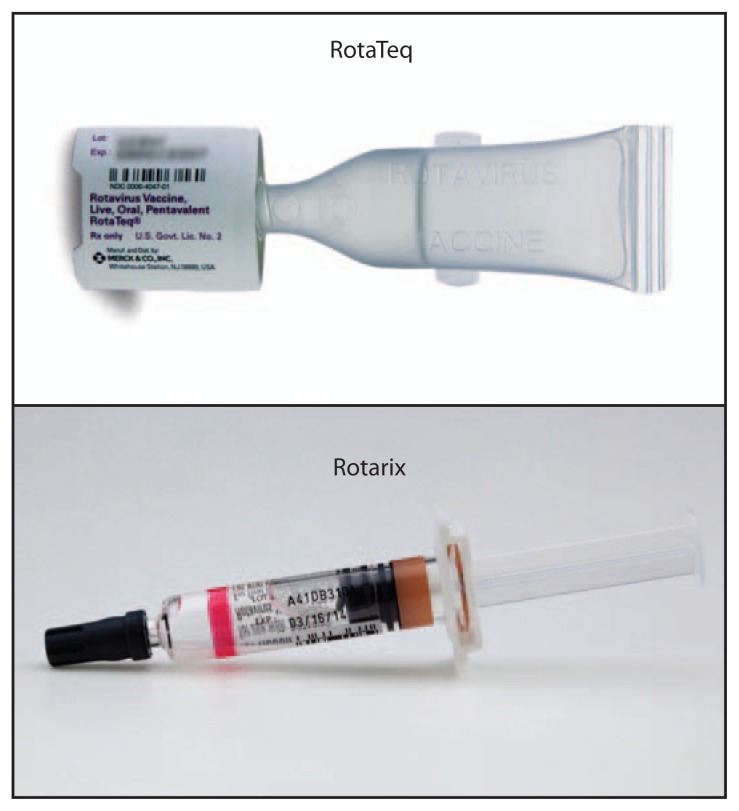
FIGURE.
Two live rotavirus oral vaccines (RotaTeq and Rotarix)*
Photos/Merck & Co., Inc. (RotaTeq) and GlaxoSmithKline Biologicals (Rotarix)
* During the period January 1, 2006–August 1, 2013, a total of 66 reports of rotavirus vaccine administration errors were submitted to the Vaccine Adverse Event Reporting System, including 39 reports of administration by injection (six for RotaTeq and 33 for Rotarix), of which nine reports included an explanation for the error, which included the following: misinterpreting package insert instructions, confusing the Rotarix oral applicator syringe with a syringe for injection, confusing the Rotarix vial (not pictured) with a vial used for injectable vaccine, inadequate training, and not reading the package insert.