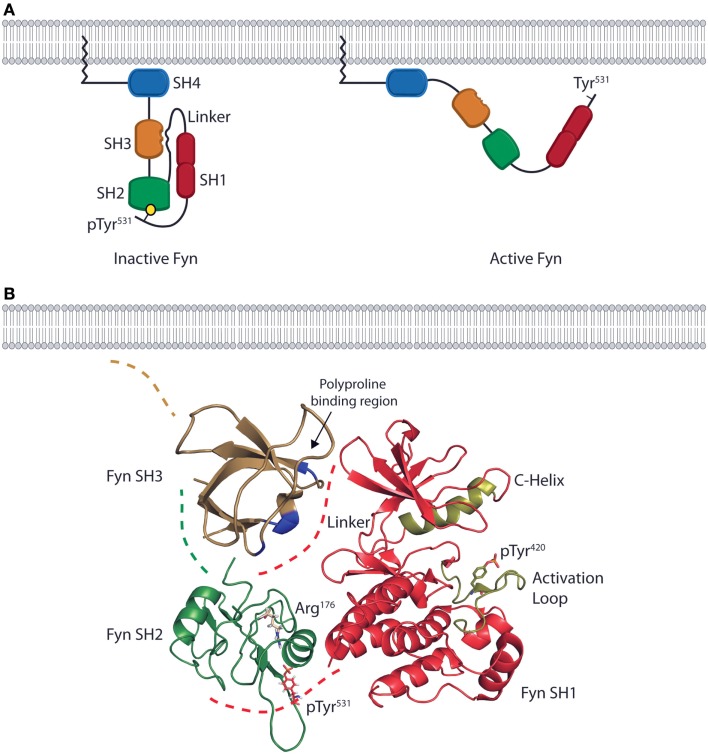
Figure 1.
The domain structure and intramolecular interactions of the Fyn kinase. (A) Models of the two primary conformations of the Fyn kinase. The domain structure of Fyn consists of four SH domains. The relative positions of the domains are shown in both the inactive (left) and active (right) states. The N-terminal SH4/unique domain (blue) lies proximal to the membrane-anchoring myristoylation site. The SH4 domain is followed by the SH3 (gold) and SH2 (green) domains responsible for mediating interactions between Fyn and its target substrates. These domains are followed by a linker region that connects the SH2 domain to the bilobal SH1/kinase domain (red) responsible for enzymatic activity. The inactive form of the kinase is kept in a closed conformation by intramolecular interactions between the SH2 domain and a C-terminal phosphotyrosine as well as by an interaction between the SH3 domain and a polyproline helix in the linker region (left). The active conformation is adopted upon disruption of these interactions and opening of the kinase. In the active conformation, the SH2 and SH3 domains mediate protein–protein interactions, while the kinase domain phosphorylates downstream effectors (right). (B) Ribbon diagrams of the SH3, SH2, and SH1 domains show their relative positions in the inactive conformation. Positions are based on the structure of auto-inhibited Src (PDB deposition 2SRC), which has an identical domain arrangement. The blue high-lighted area of the SH3domain is the site of interaction with the linker region. The SH2 domain of Fyn (green) possesses an Arg residue in the second β-sheet (ArgβB5, Arg176) that is conserved across SFK family members. This Arg residue is the primary site of interaction with the phosphotyrosine on target substrates. In the inactive conformation, Arg176 is bound to the C-terminal inhibitory pTyr531 of Fyn. These intramolecular interactions place the SH2 and SH3 domains in a position to occlude the kinase domain (red) from substrate binding, thus preventing phosphate transfer. The ribbon diagram of the kinase domain is derived from PDB deposition 2DQ7, and the positions of the C-Helix and activation loop (gold) show the structure of the Fyn kinase domain complexed with staurosporine. To date, this is the only conformation of the Fyn kinase domain that has been characterized, and it is used here to demonstrate the intramolecular interactions that keep Fyn in an inactive state. The SH3 domain ribbon diagram is from PDB deposition 1NYG, and the SH2 ribbon diagram is from the PDB deposition 1AOT, and is representative of the SH2 domain bound to a phosphotyrosine-containing peptide.