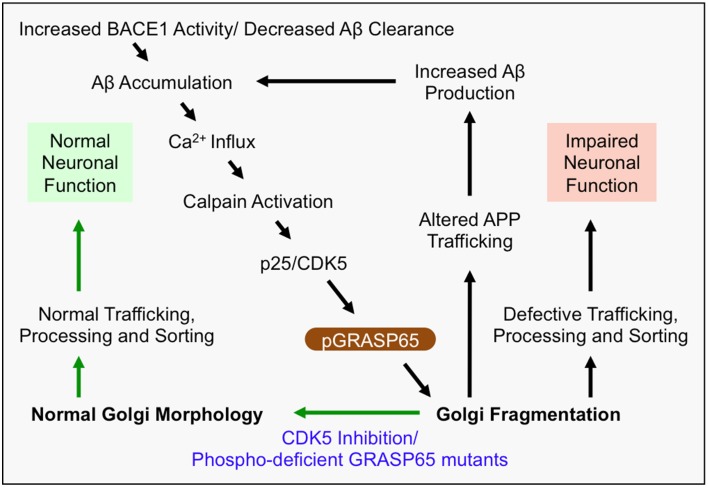
Figure 2.
Mechanism of Golgi defects in AD. Increased BACE1 activity and/or decreased Aβ clearance from the extracellular space leads to the accumulation of Aβ. In turn, Aβ induces Ca2+ influx, which activates Calpain and induces cleavage of p35 to p25. Consequently, p25 activates CDK5, which in turn phosphorylates and inactivates the Golgi structural protein GRASP65 (i.e., pGRASP65). Inactivation of GRASP65 causes Golgi fragmentation, which alters trafficking of APP, and potentially the secretases, leading to increased Aβ production. This deleterious feedback loop (indicated by black arrows) would impair the integrity of the secretory pathway for sorting, trafficking and modifications of many essential proteins, which may compromise neuronal function, activate inflammatory responses, or cause neuronal cell death. Inhibition of CDK5 or expression of non-phosphorylatable GRASP65 mutants restores the normal Golgi morphology and reduces APP trafficking and Aβ production (indicated by green arrows). Therefore, rescue of the defective Golgi may delay AD development.