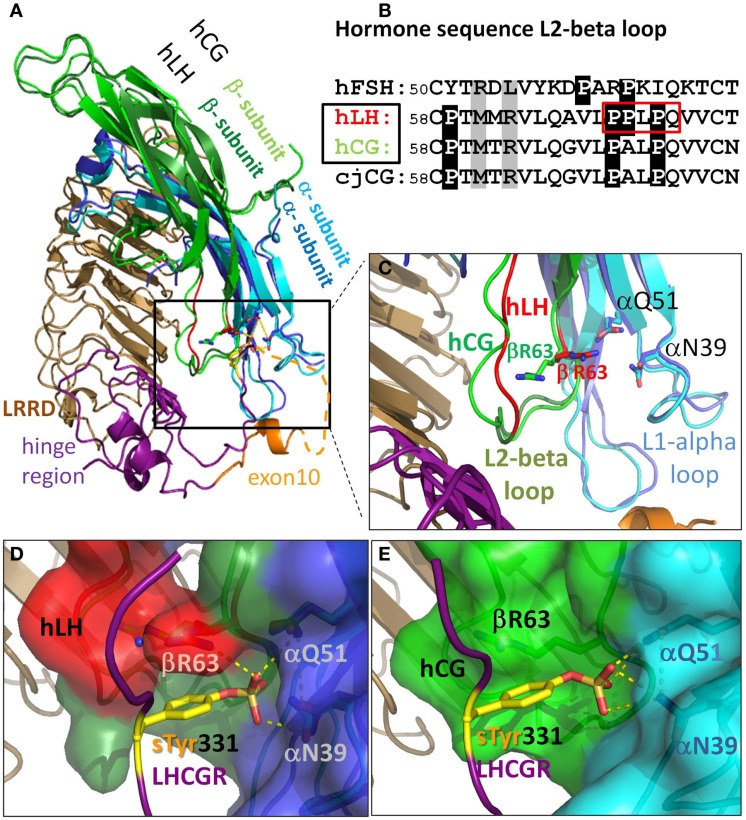
Figure 3.
There are differences in the L2-beta loop for hLH and hCG. This gives rise to tighter binding for hLH than for hCG at sTyr331, the second binding site at LHCGR. (A) Homology model details of superimposed hormones hLH and hCG extracellularly bound to LRRD and hinge region of LHCGR based on the crystal structure of FSH/FSHR [4AY9, Ref. (9)]. (B) Sequence differences in L2-beta loop of these glycoprotein hormones (cjCG: New world monkey Callithrix jacchus). Gray: positions representing interacting elements of the sTyr moiety of the LHCGR hinge region. White on black background: differences in prolines between FSH and hLH, hCG, and even between hLH (red boxed) and hCG. (C) hLH was modeled based on the hCG structure (PDB: 1HRP, light green). Close up view: the L1-alpha loop is similar for hLH (dark blue) and hCG (cyan). The differing conformations of the L2-beta loop for hLH is based on a fragment in the crystal structure of Insulysin (3TUV) with identical sequence PPLPQ. The resulting backbone conformation of hLH (red) clearly differs from that in hCG structure (light green). The additional proline in hLH (see red box in B) restricts the conformational degree of freedom of βR63 (red) located in the N-terminal flank of the L2-beta loop. (D) Detailed view for hLH. The resulting binding cavity for sTyr331 formed by the L1-alpha loop (dark blue surface) and L2-beta loop (red surface) is more surrounded in hLH, and is flanked by the βR63 (red) and thus provides an additional H-bond donor for the interaction with the sTyr331 (yellow) of LHCGR compared to (E) detailed view for hCG (light green), where βR63 does not interact with sTyr331 and the binding pocket is much wider.