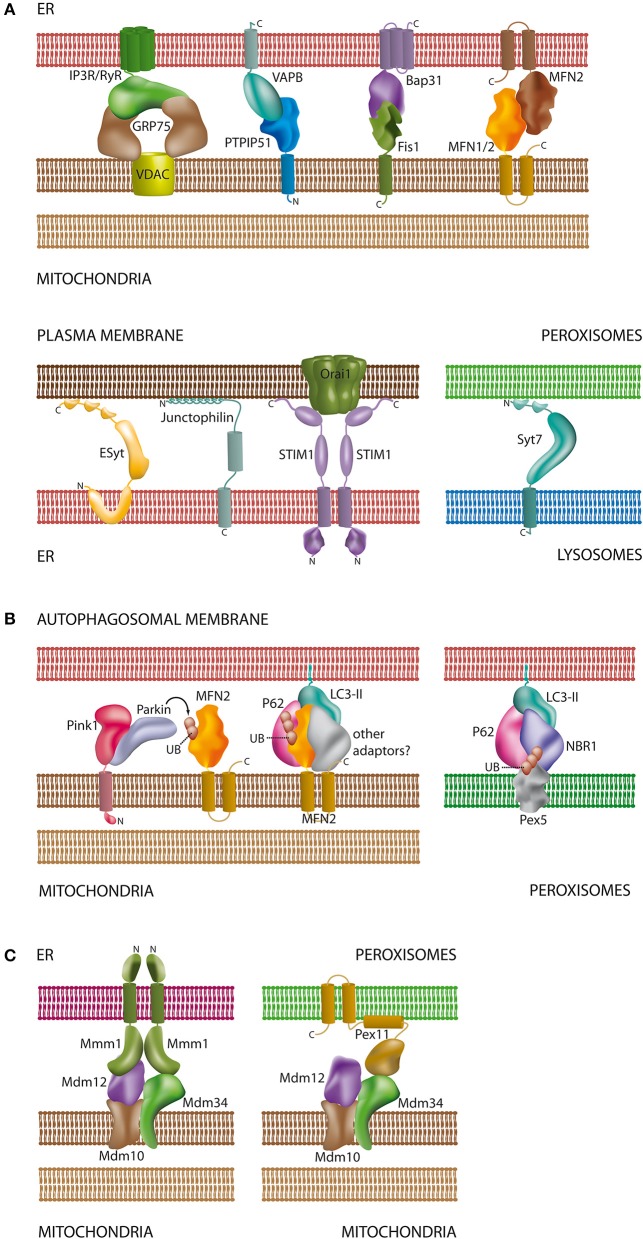
Figure 2.
Schematic overview of proteins and lipids involved in the interaction of organelles. (A) Tethering complexes in mammals: unlike in yeast species only a few protein complexes have been characterized at the molecular level and involve protein-protein and protein-lipid contacts [see Sections Connections between the ER and the Plasma Membrane, The Mitochondria-associated Membrane of the ER (MAM), Interplay between Peroxisomes and Mitochondria, and Lysosomal Interactions and Autophagy]. Part of the tethering complexes shown may only comprise core complexes, which will interact with additional proteins for regulatory purposes; (B) contacts between mitochondria/peroxisomes and the autophagosomal membrane: both organelles require ubiquitination of membrane proteins for recognition by the autophagosome. In addition to MFN2 (Mitochondria) and Pex5 (Peroxisomes) other ubiquitinated organelle proteins have been described to participate in autophagosomal contacts [see Sections The Mitochondria-associated Membrane of the ER (MAM) and Lysosomal Interactions and Autophagy]; (C) ERMES as a multifunctional tethering complex in yeast: unlike mammals, yeast species possess the ERMES oligomeric complex at the mitochondrial membrane. ERMES forms complexes with the ER and peroxisomes [see Sections The Mitochondria-associated Membrane of the ER (MAM) and Interplay between Peroxisomes and Mitochondria]. In addition, a considerable number of other tethering complexes (not shown) have been described in yeast (Prinz, 2014). For molecular details and references of the depicted complexes please refer to the corresponding sections of this review. Membrane spanning α-helices in the proteins are depicted as cylindrical segments; C- and N-termini are marked with the corresponding letters.