Abstract
Interferon-based therapies were until recently the standard of care in chronic hepatitis C (CHC), despite their side effects. We aimed to review the available data on the depression and suicide in CHC patients receiving or not antiviral therapy based on interferon treatment. A PubMed search was performed, identifying relevant papers published between 1991 and January 2015 concerning major depressive disorders and suicidal risk in patients under interferon. A total of 21 relevant papers were retrieved. Prospective studies reported depression as the most common side effect of interferon, with an incidence peak between weeks 8 and 12 of therapy. Suicide risk was analyzed in 7 of the reviewed articles, and case reports of attempted suicide were discussed. Moreover, studies have shown that pretreatment with serotonin reuptake inhibitors is a good strategy for the prevention of interferon-induced depression.
Keywords: Depression, antiviral therapy, pegylated interferon, ribavirin, suicide, chronic hepatitis C, neuropsychiatric events
Introduction
Hepatitis C virus (HCV) infection is one of the main causes of chronic liver disease worldwide, affecting an estimated 170 million people [1,2]. Current standard of care for the treatment of chronic hepatitis C (CHC) infection was until recently represented by the combination of pegylated interferon α (peg-IFN-α) and ribavirin (RBV) for either 24 or 48 weeks depending on genotype. Until the approval of sofosbuvir, simeprevir and daclatasvir in 2014 as IFN-free regimens, even after the introduction in 2011 of the first generation direct acting antivirals, namely boceprevir and telaprevir, IFN was also used as a component of a triple combination regimen. However, by reasons regarding the costs of therapy, there are many countries worldwide where IFN therapy is still available as therapy for CHC, or patients still continuing the therapy. The primary goal of CHC therapy is sustained virological response (SVR), defined as an undetectable HCV-RNA level (<50 IU/mL) 24 weeks after treatment withdrawal.
Although SVR is obtained in 46% of cases in patients infected with genotype 1, and, respectively 76% in patients infected with genotypes 2 and 3, the combination therapy with peg-IFN-α therapy and RBV is often discontinued because of significant neuropsychiatric adverse events which may limit its use. The most common psychiatric side effect is depression, with the prevalence ranging from 30% to 70% [2]; consequently, depending on the gravity of depressive disorder, psychosis, suicidal ideation, and suicide attempt may occur [3-5]. Studies focusing on the rate of SVR and factors associated with SVR show that severe depression leading to suicide ideation/suicide attempt is also the reason for early termination of antiviral treatment in CHC patients [6]. These data imply a proper management and prevention strategies of depression in these patients according to current guidelines. Depressive symptoms occur mostly in the early stages of treatment and reach a peak between 4 and 16 weeks [4].
Given the fact that there is an increased prevalence of psychiatric comorbidity in CHC patients compared to the general population [7], and that psychiatric disorders represent a contraindication to the antiviral therapy, an exhaustive review of the psychiatric side effects occurring during peg-IFN-α therapy is useful. Mental health issues may also reduce quality of life and interfere with treatment compliance.
In this paper we review the studies related to major depressive disorders and its most severe consequences suicide ideation and suicide attempts in CHC patients undergoing antiviral treatment (peg-IFN-α in association with RBV), we present the main pathophysiological mechanisms of IFN-α-induced depression, and discuss prevention versus management strategies during and after cessation of IFN-α therapy. We also briefly report on the incidence of depression among untreated CHC patients, for better understanding of the effects of IFN-induced metabolic changes in the context of the already affected central nervous system in CHC patients. This information also serves as a “control” to the data on treated patients.
Materials and methods
A PubMed search of all published articles on psychiatric adverse events of IFN-α therapy in CHC patients was carried out. We limited our search to articles published between 1991 (since IFN therapy for CHC had been first approved) and January 2015. We focused on articles concerning the topic of major depressive disorders and suicidal risk; the search included the terms: major depression, major depressive disorders, interferon alpha therapy, IFN alpha, CHC, suicide, suicide risk, suicide ideation. Only articles in English and French were included. The titles and abstracts were examined and after obtaining full-text articles of potentially relevant studies, inclusion and exclusion criteria were applied. We herein included prospective studies reporting the incidence of IFN-α-related major depressive disorders, suicide ideation, or suicide attempt; and observational studies estimating frequency of depression in CHC patients under IFN-α treatment.
We excluded studies regarding the onset of depression in CHC patients receiving antiviral treatment other than IFN-α (IFN-free regimens), other neuropsychiatric side effects than major depression (mood alteration, anxiety, cognitive impairment and somatic symptoms), and studies focusing on treatment and prevention of IFN-α-induced depressive disorders.
The majority of papers included in this review analyze the prevalence of major depressive disorder related to antiviral therapy (IFN-α monotherapy or in combination with RBV).
Results
Using PubMed database, 356 articles were identified and titles and abstracts were examined. At this stage 319 were excluded because they did not meet the inclusion criteria. We further excluded 18 case reports and articles not serving the purpose of this paper. After we examined the references of remaining articles we obtained 21 relevant papers (Fig. 1). Selected articles were reviewed and we summarized in (Table 1) the following data: author, year of publication, number of patients included in the study, dose and type of IFN-α, and adjunctive RBV, duration of antiviral treatment, incidence of major depressive disorder and suicide ideation/suicide attempts when reported, scales and methods of assessment of depression, and risk factors for depression.
Figure 1.
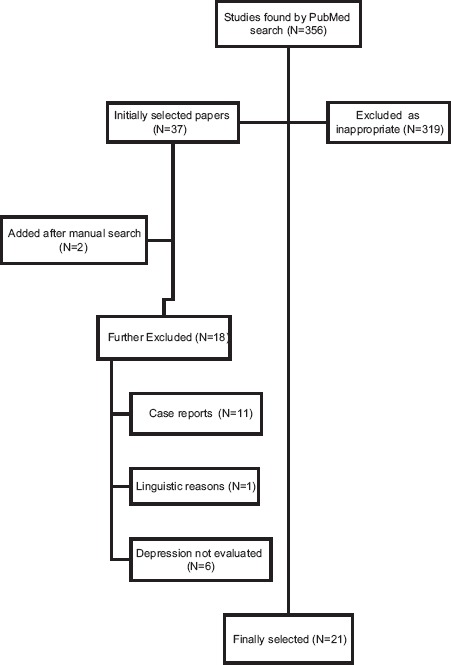
Flow diagram of article selection for the incidence of major depressive disorders
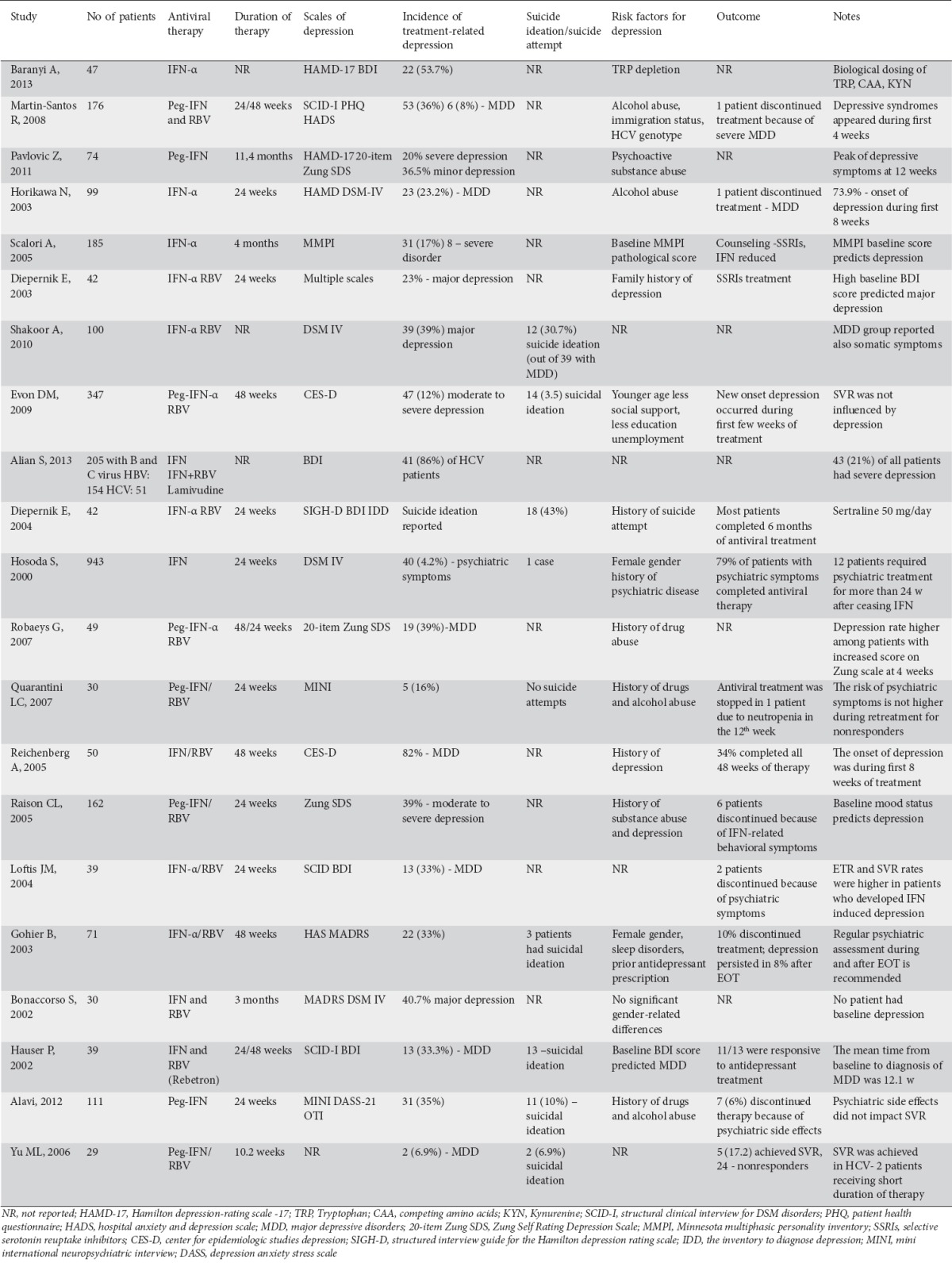
Table 1.
Prevalence of interferon (IFN)-induced depression and suicide risk
Depression and suicide risk in untreated CHC patients
Psychiatric disorders are reported to be frequently associated in patients with CHC and HIV infection [7]. Psychiatric comorbidities are also more commonly reported among CHC patients compared to the general population [2,8]. This is considered to be related to a personal history of high-risk behavior, such as intravenous drug abuse, alcohol abuse, high-risk sexual behavior; in intravenous drug users, an increased incidence of psychiatric disorders (98%) and suicide risk has been reported [7,9]. US veterans were repeatedly studied regarding psychiatric comorbidities in relation to HCV infection. The study concerning the most extensive veteran population [10] pointed out that 85% of the 33,842 veterans recruited had a personal history of psychiatric disorder or drug abuse.
Stigmatization and the psychosocial impact of a personal history of CHC infection increase the rate of depression among these patients. A study by Alavi et al [11], designed to evaluate depression prior and during treatment in 163 CHC patients, showed a rate of major depression of 36% (57 patients) and a moderate to severe suicide risk of 18% (28 patients) before treatment; depression and suicide risk were assessed by depression anxiety stress scale (DASS) score. Potential risk factors associated with depression at enrollment included unstable employment, recent injecting drug use, and poor/limited social activity.
There is also evidence that HCV infection may directly or indirectly induce biological changes in the central nervous system, which may trigger psychiatric disorders. Studies using proton magnetic resonance spectroscopy showed a modified creatine/choline ratio, N-acetyl-aspartate/creatine ratio, myo-inositol/creatine ratio and also an altered metabolism of these substances, changes consistent with cerebral immune activation [2]. It has also been reported that decreased dopamine and serotonin transporters binding is associated with cognitive impairment in CHC patients [2]. Pathophysiological pathways of this mechanism have not been completely understood yet, but there is recent data suggesting the possibility of HCV spreading into the central nervous system where it replicates at a low level [7].
Depression and suicide risk in CHC patients under peg-IFN-α monotherapy or in combination with RBV
Suicide and suicide attempts during IFN-α therapy have rarely been reported mainly as isolated case reports [12-14]. Most of the studies discussing the topic of neuropsychiatric side effects of IFN-α therapy describe mainly the prevalence of depression, assessed by different scales; there are, however, a few studies regarding major depression complicated by psychotic episodes and suicidal ideation.
More specifically, Reichenberg et al [15] assessed the rates of depressive symptoms and cognitive impairment in 50 CHC patients treated with peg-IFN plus RBV over a period of 72 weeks (48 weeks of treatment and 24 weeks of post-treatment follow up); 82% of patients developed major depressive symptoms according to the Center of Epidemiologic Studies – Depression (CES-D) scale. Patients were evaluated by CES-D at baseline, at weeks 1, 2, and 4, every 4 weeks until cessation of the treatment, and at weeks 4, 8 12, and 24 after termination of treatment.
Dieperink et al studied the occurrence of suicidal ideation in 55 consecutive CHC veterans treated with IFN-α and RBV [4]. Thirteen of the 55 veterans were part of the control group, and 42 patients received antiviral treatment. Patients undergoing IFN-α and RBV treatment filled in depression evaluating scales (SIGH-D, Structured Interview Guide of the Hamilton Depression Scale; BDI, Beck Depression Inventory) at baseline and at weeks 4, 8, 12, and 24. Study results show a rate of suicidal ideation during treatment presenting in 26% (11) of patients; 45% (n=5) of them required antidepressants. No patient attempted suicide during the course of treatment.
A multicenter prospective cohort study conducted by Alavi et al [11] evaluated the impact of IFN-α therapy (monotherapy) on mental health in 163 CHC patients with personal history of injection drug use, of which 111 were treated. Depressive disorder and suicide risk were evaluated prior and during (new onset) treatment by questionnaires [mini international neuropsychiatric interview (MINI); and depression anxiety stress scale (DASS)]. Of the 111 who received antiviral treatment, 88 did not have depression at study enrollment. Of these 88 participants, 31 (35%) developed new-onset depression during treatment, and 11 of 31 (35%) of these received antidepressants. Suicide risk was classified in 3 categories according to MINI, i.e. low, moderate, and high; the highest rate of suicidal behavior was reported in week 12 of treatment (34%). Overall, 6% of participants discontinued therapy early due to psychiatric side effects. In addition, it was shown that mental health parameters (depression and/or suicide ideation) at enrollment or during treatment do not impact SVR.
A large prospective analysis conducted by Evon et al [16] (Virahep-C study) analyzed the effects of pre-existing depressive symptoms and occurrence of new onset depression during antiviral therapy in a sample of 394 patients. At baseline, 12% of patients had a depressive disorder, 13% were using antidepressants and no suicide ideation was reported. The incidence of new-onset depression was 26% during first 24 weeks of treatment; 4% of participants reported suicidal or homicidal ideation but without any attempts. Baseline patient characteristics which predicted new onset depression included: younger age, less social support, lower education, unemployment, and antidepressant use. The study also demonstrated no statistically significant association between incident depression and SVR rates.
Another study conducted by Barany et al suggested a biopsychosocial model of adverse events of IFN-α therapy in CHC patients, and reported new-onset depressive symptoms at a rate of 53.7% (22 of 41 patients) [17]. The study offers an insight to the pathophysiology of IFN-induced depression showing a significant enhancement of indoleamine 2,3-dioxygenase activity and an increase of kynurenine (KYN) neurotoxic metabolites during antiviral treatment. Another major finding of the study is that, as shown by the tryptophan (TRP)/competing amino acids quotient, TRP availability to the brain declines in patients with IFN-α treatment-related depressive symptomatology, suggesting a need of higher TRP to stabilize their mood.
A recent study conducted by Alian et al [18] analyzing the correlation of depression with hepatitis drugs in 205 patients with chronic hepatitis B and C, reported a prevalence of depression among IFN-treated patients of 100% vs. 94.4% in patients receiving IFN plus RBV treatment. Depression was also more frequent in CHC than chronic hepatitis B patients (86.3% vs. 68.8%, respectively).
A temporal analysis of symptoms occurring during IFN-α therapy revealed different times of onset for the neuro-vegetative/somatic vs. mood/cognitive symptoms. Different symptoms (fatigue, decreased appetite, and pain) develop at early stages of treatment, during the first weeks of therapy and persist throughout the whole duration of therapy [19]. Cognitive impairment and mood disorders (depressive symptoms, anhedonia, memory disturbances) develop at later stages of treatment (after week 4), with an increasing intensity of depressive symptoms after 8 weeks of therapy.
The study led by Diepernik et al which examined the occurrence of suicide ideation among patients untreated and treated with IFN-α2b, showed a peak of suicide ideation during weeks 8 and 12 [4]. As already mentioned, Alavi et al, who examined the impact of IFN-α2a treatment on the mental health during recent HCV infection, considered that the maximum suicidal risk occurs during week 12 of treatment [11]. The prospective 72-week study by Reichenberg et al, discussed previously, outlined an increase in depressive symptomatology over the first 8 weeks of treatment, with a minor decrease after week 8, and increasing again in the last 12 weeks of treatment; 39% of patients developed new-onset major depressive disorder by week 8, 52% during the 28th week of treatment and 55% during week 40.
Most neuropsychiatric side effects resolve after the end of treatment; however, cases of persistent or newly developed major depressive disorders associated with suicide attempts occurring after cessation of therapy have been reported. Riflet et al published 5 cases of suicide attempts in CHC patients treated with IFN-α; in case of two of these patients depressive symptoms did not resolve after the end of therapy, and another two patients developed depressive disorder after cessation of IFN-α treatment [12].
Pathophysiology of IFN-induced depression
The pathophysiology of neuropsychiatric side effects occurring during IFN-α therapy, although not completely elucidated yet, involves two main mechanisms: impairment of monoamines metabolism and neuroendocrine dysfunction [2]. Recent studies showed that immune mediators, including IFN-α, induce significant changes in enzymatic pathways involved in monoamines metabolism (serotonin, dopamine, norepinephrine). These alterations imply the activation of indoleamine-2,3-dioxygenase which rushes TRP catabolism, decreases 5-hydroxytryptamine (5-HT) serum levels, following an increase in 5-HT neurotoxic metabolites: KYN and quinolinic acid [2,8,20]. These changes have been shown to correlate with the onset of depressive symptomatology; moreover, there is evidence of a normalization of TRP and KYN serum levels 6-12 months after cessation of IFN-α therapy which correlates with the resolution of depressive symptoms [21]. An alteration of cerebrospinal fluid levels of 5-hydroxyindoleacetic acid during IFN-α therapy has also been observed, associated with increased rates depressive disorders [9].
Another mechanism which may explain neuropsychiatric adverse events of IFN-α therapy involves changes in the serum levels of adrenocorticotropic hormone (ACTH) and cortisol. A prospective study conducted by Raison et al examined the diurnal activity of the hypothalamic-pituitary axis and proinflammatory cytokines in 33 CHC patients prior and 12 weeks following IFN-α and RBV therapy. Depressive symptomatology was also evaluated by Montgomery-Asberg Depression Rating Scale. Results of the study showed a flattening of ACTH levels and a decrease in cortisol concentration during the day, and a nocturnal peak of cortisol and ACTH levels, which correlated with an increase in depressive symptomatology and fatigue [22].
Psychiatric management of CHC patients treated with IFN-α
Psychiatric disorders occurring during IFN-α therapy, mostly major depressive disorders, may lead to suicide ideation and/or suicide attempts; this is why a thorough psychiatric evaluation, including depression assessment scales or criteria, namely International Classification of Diseases (ICD-10) and The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), are required before the initiation of IFN therapy to identify high-risk patients. Predictive factors for IFN-α-induced depression include: personal history of depressive disorder, family history of psychiatric disorders, early onset of symptoms (sleep disturbance, loss of appetite), baseline stress, lack of social support, depression during previous IFN treatment, and increased serum levels of proinflammatory cytokines (interleukins -10 and -6, and tumor necrosis factor-receptor-1) [2,17].
The ELPA (European Liver Patient’s Organization) organized a European expert conference (2011 EASL meeting) to review the medical literature concerning the management of psychiatric disorders in CHC patients and to develop expert recommendations. It is recommended to perform a detailed psychiatric evaluation of CHC patients prior to introducing IFN-α therapy, which includes personal history of psychiatric comorbidity, addictive behavior, previous suicide attempts, and psychosocial functioning; it is also required to inform the patient about the neuropsychiatric adverse events likely to occur during IFN-α therapy. Close monitoring should be performed every 4, 12 weeks, or even more frequently (every 2-4 weeks) when required (new-onset mood disorders, drug/alcohol abuse, personal history of psychiatric comorbidity). Considering that suicide risk is the highest during first 12 weeks of therapy, closer follow up is recommended during this interval.
The pharmacological management of adverse events depends on the time when they occur. Sleep disturbance, as a risk factor for depressive symptoms occurrence, requires early therapeutic management. Data shows that 10-14% of patients discontinue IFN-α therapy because of fatigue, mood disorders, and sleep disturbance [9]. Regarding the efficacy of the antidepressants, studies have shown that selective serotonin re-uptake inhibitor (SSRI) therapy decreased the severity of depressive symptoms by 50%, with an antidepressant treatment response rate of more than 85% after 5-6 weeks of therapy [23]. It is also recommended that antidepressant therapy to continue 6 to 12 weeks after IFN-α cessation therapy because of the risk of depressive disorders, suicide ideation or cognitive disorders recurrence [2,9,12]. However, initiation of antidepressant therapy should consider drug-drug interactions, underlying hepatic function, the possibility of drug induced hepatotoxicity and other adverse side effects [24].
Prevention of IFN-α-induced depression
Given the evidence of decreased serotoninergic neurotransmission as a pathophysiological mechanism of IFN-α-induced depression, the administration of SSRIs was considered as a strategy for treatment and prevention of IFN-α-induced depression. In a study led by Schaefer et al [25] on 14 psychiatric CHC patients receiving prophylactic treatment with citalopram (20 mg/day) before and during IFN-α therapy, a lower incidence of major depressive disorder was reported compared to the two control groups (CHC patients with and without psychiatric disorders who did not receive prophylactic antidepressant therapy). Major depression during the first 24 weeks of IFN-α therapy was 14% in the group who received citalopram vs. 64% and 55% in the control groups. Another study focused on 10 patients with previous history of IFN-α-induced depression, who received antiviral re-treatment with peg-IFN and RBV and prophylactic SSRIs 3 weeks before the initiation of antiviral treatment, reported a lower incidence of IFN-induced depression compared to the first therapeutic regimen [26]. There are also several prospective trials which show contradictory data [27-30], merely due to methodological issues: small groups of patients, lack of consistent follow up (one study evaluated depression only for the first 3 months of antiviral treatment). However, in spite of the heterogeneous data, antidepressive prophylaxis (escitalopram, citalopram and paroxetine) was reported to be well tolerated and does not affect SVR.
Antidepressant prophylaxis must be continued during the course of antiviral therapy and up to 12 weeks after discontinuation. According to the expert consensus statement (EASL 2012), not every CHC patient undergoing antiviral therapy should be administered antidepressant pretreatment; every case must be evaluated in a customized fashion [2]. This approach would improve the quality of life of CHC patients, impaired before but also under IFN therapy [31-33].
The advent of non-IFN regimens is expected to decrease the number of psychiatric side effects including suicide.
Limitations of the study
In present there is considerable variability of frequency and extent of IFN-induced depression reported in different cohorts of patients. A recent study conducted by Schafer et al [34] systematically reviewed recent literature to identify to what extent methodological bias contributed to inconsistent results in different studies. The results showed that variability of findings is mainly due to different study populations, treatment regimens (IFN monotherapy vs. IFN plus RBV), methodological approaches and different screening instruments for depression, particularly cut-off criteria for clinically relevant depression. Thus, inconsistency of results between studies may be justified. Further research in this field necessitates the use of standardized assessment and objective criteria and guidelines in clinical practice for treatment of IFN-induced depression. Although IFN is no more recommended for CHC therapy [35] in many countries it is still the available alternative and there are patients still continuing IFN therapy. Knowing the depressive and suicidal side effects of IFN is still important.
Concluding remarks
Psychiatric side effects associated with IFN-α therapy in CHC patients are the main cause of antiviral treatment discontinuation, which results into a decreased rate of SVR. By far, the most common side effect of IFN-α therapy is depression; major depression disorder and new-onset psychosis may lead to suicide ideation and suicide attempt. Given this fact, a careful psychiatric evaluation is required prior to initiation of IFN therapy to identify high-risk patients. Therefore, in selected cases antidepressant therapy as a prophylactic measure should be considered.
Biography
Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca, Romania
Footnotes
Conflict of Interest: None
References
- 1.EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer M, Capuran L, Friebe A, et al. Hepatitis C infection, antiviral treatment and mental health: A European expert consensus statement. J Hepatol. 2012;57:1379–1390. doi: 10.1016/j.jhep.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Silverman BC, Kim AI, Freudenreich O. Interferon induced psychosis as a “psychiatric contraindication” to hepatitis C treatment: a review and case-based discussion. Psychosomatics. 2010;51:1–7. doi: 10.1176/appi.psy.51.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML. Suicidal ideation during interferon-α2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26:237–240. doi: 10.1016/j.genhosppsych.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Negro F. Adverse effects of drugs in the treatment of viral hepatitis. Best Pract Res Clin Gastroenterol. 2010;24:183–192. doi: 10.1016/j.bpg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Yu ML, Dai CI, Lee LP, et al. Outcome of chronic hepatitis C patients who required early termination of pegylated interferon-alpha plus ribavirin combination therapy. Antivir Ther. 2006;11:1015–1019. [PubMed] [Google Scholar]
- 7.Modabbernia A, Poustchi H, Malekzadeh R. Neuropsychiatric and psychosocial issues of patients with hepatitis C infection: a selective literature review. Hepat Mon. 2013;13:e8340. doi: 10.5812/hepatmon.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristiansen MG, Lochen ML, Guttberg TS. Total and cause-specific mortality rates in a prospective study of community acquired hepatitis C virus infection in northern Norway. J Viral Hepat. 2011;18:237–244. doi: 10.1111/j.1365-2893.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 9.Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18:153–160. doi: 10.1111/j.1365-2893.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- 10.El-Seraq HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123:476–482. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- 11.Alavi M, Grebely J, Matthews GV, et al. Impact of pegylated interferon alfa-2a treatment on mental health during recent hepatitis C virus infection. J Gastroenterol Hepatol. 2012;27:957–965. doi: 10.1111/j.1440-1746.2011.07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rifflet H, Vuillemin E, Oberti F, et al. Suicidal impulses in patients with chronic viral hepatitis C during or after therapy with interferon alpha. Gastroenterol Clin Biol. 1998;22:353–357. French. [PubMed] [Google Scholar]
- 13.Fabregas BC, Moura AS, Marciano RS. Clinical management of a patient with drug dependence who attempted suicide while receiving peg interferon therapy for chronic hepatitis C. Braz J Infect Dis. 2009;13:387–390. doi: 10.1590/S1413-86702009000500015. [DOI] [PubMed] [Google Scholar]
- 14.Janssen HL, Brower JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 15.Reichenberg A, Gorman GM, Dieterich D. Interferon-induced depression and cognitive impairment in Hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19(Suppl. 3):174–178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- 16.Evon DM, Ramcharran D, Belle SH, Terrault NA, Fontana RJ, Fried MW Virahep-C Study Group. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: results of the Virahep-C study. Am J Gastroenterol. 2009;104:2949–2958. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- 17.Barany A, Meinitzer A, Stepan A, et al. A biopsychosocial model of interferon-alpha-induced depression in patients with chronic hepatitis C infection. Psychother Psychosom. 2013;82:332–340. doi: 10.1159/000348587. [DOI] [PubMed] [Google Scholar]
- 18.Alian S, Masoudzadeh A, Khoddad T, Dadashian A, Ali Mohammadpour R. Depression in hepatitis B and C, and its correlation with hepatitis drugs consumption (interferon/lamivudine/ribavirin) Iran J Psychiatry Behav Sci. 2013;7:24–29. [PMC free article] [PubMed] [Google Scholar]
- 19.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 20.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 21.Zinego AL, Cozzi A, Carpenedo R, et al. HCV patients, psychopatology and tryptophan metabolism: analysis of the effects of pegylated interferon plus ribavirin treatment. Dig Liver Dis. 2007;39:107–111. doi: 10.1016/s1590-8658(07)80021-1. [DOI] [PubMed] [Google Scholar]
- 22.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflamatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 24.Horikawa N, Yanozaki T, Izumi N, Uchiihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. Gen Hosp Psychiatry. 2003;25:34–38. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42:793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Kraus MR, Schafer A, Al-Taie O, Scheurlen M. Prophylactic SSRI during interferon alpha re-therapy in patients with chronic hepatitis C and a history of interferon-induced depression. J Viral Hepat. 2005;12:96–100. doi: 10.1111/j.1365-2893.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 27.Diez-Quevedo C, Masnou H, Planas R, et al. Prophylactic treatment with escitalopram of pegylated interferon alfa-2a-induced depression in hepatitis C: a 12-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2011;72:522–528. doi: 10.4088/JCP.09m05282blu. [DOI] [PubMed] [Google Scholar]
- 28.Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 29.Morasco BJ, Rifai MA, Loftis JM, Indest DW, Moles JK, Hauser P. A randomized trial of paroxetine to prevent interferon-alpha induced depression in patients with hepatitis C. J Affect Disord. 2007;103:83–90. doi: 10.1016/j.jad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51:401–408. doi: 10.1176/appi.psy.51.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakoor A, Shafgat F, Mehmud TE, et al. Frequency of depression and somatic symptoms in patients on interferon alpha/ribavirin for chronic hepatitis C. J Ayub Med Coll Abbottabad. 2010;22:6–9. [PubMed] [Google Scholar]
- 32.Pojoga C, Dumitraşcu DL, Pascu O, Grigorescu M, Radu C, Damian D. Impaired health-related quality of life in Romanian patients with chronic viral hepatitis before antiviral therapy. Eur J Gastroenterol Hepatol. 2004;16:27–31. doi: 10.1097/00042737-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Pojoga C, Dumitraşcu DL, Pascu O, Grigorescu M. The effect of interferon alpha plus ribavirin on health-related quality of life in chronic C hepatitis. The Romanian experience. J Gastrointestin Liver Dis. 2006;15:31–35. [PubMed] [Google Scholar]
- 34.Schafer A, Wittchen HU, Seufert J, Kraus MR. Methodological approaches in the assessment of interferon-alpha-induced depression in patients with chronic hepatitis C – a critical review. Int J Methods Psychiatr Res. 2007;16:186–201. doi: 10.1002/mpr.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. EASL Recommendations on treatment of hepatitis C 2015. J Hepatol. 2015 doi: 10.1016/j.jhep.2022.10.006. in press. [DOI] [PubMed] [Google Scholar]


