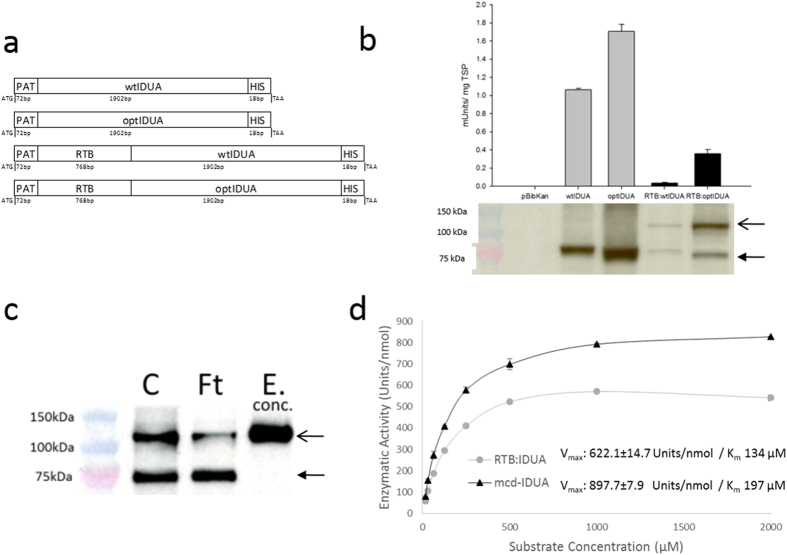
Figure 1. Production of IDUA and RTB:IDUA fusions in plants.
(a) Construct design of the recombinant proteins used for plant-based expression. PAT, plant signal peptide from potato patatin gene; RTB, RTB lectin; wtIDUA, native human alpha-L iduronidase; optIDUA, codon-optimized IDUA; HIS, 6X histidine tag. (b) IDUA yields in crude leaf extracts as assessed by IDUA enzyme activity and Western immunoblot. Analyses represent data from at least four biological replicates comparing extracts of N. benthamiana leaves harvested at the peak time-point for each construct: wtIDUA, 48 h; optIDUA, 120 h; RTB:wtIDUA, 72 h; RTB:optIDUA, 72 h; and pBibKan “empty vector” control at 72 h. For the Western blot, 40 μg total soluble protein/lane were size-separated (SDS-PAGE), transferred to nitrocellulose membranes and detected using an anti-IDUA antibody. (c) Lectin binding activity of fusion protein. Western blot analysis of key fractions before and after lactose affinity chromatography; fractions were detected using anti-IDUA antibodies: crude extract (C), flow-through fraction (Ft), and concentrated elution fraction (E-conc; concentrated 10-fold). Arrows in (b) and (c) approximate the expected sizes for full-length RTB:IDUA (open arrow; ~110 kDa) and for IDUA (closed arrow; ~75 kDa). (d) Michaelis-Menten enzyme kinetics of mcd-IDUA and RTB:IDUA purified extracts. IDUA enzyme activity was determined for various concentrations of IDUA using mammalian cell-derived IDUA (mcd-IDUA; certified as >99% purity (R&D System)) and plant-derived RTB:IDUA (purified by lactose affinity and size exclusion chromatography yielding a product of 98% purity; see Supplementary Fig. S1 online).