Abstract
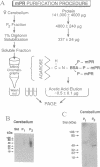
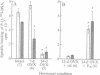
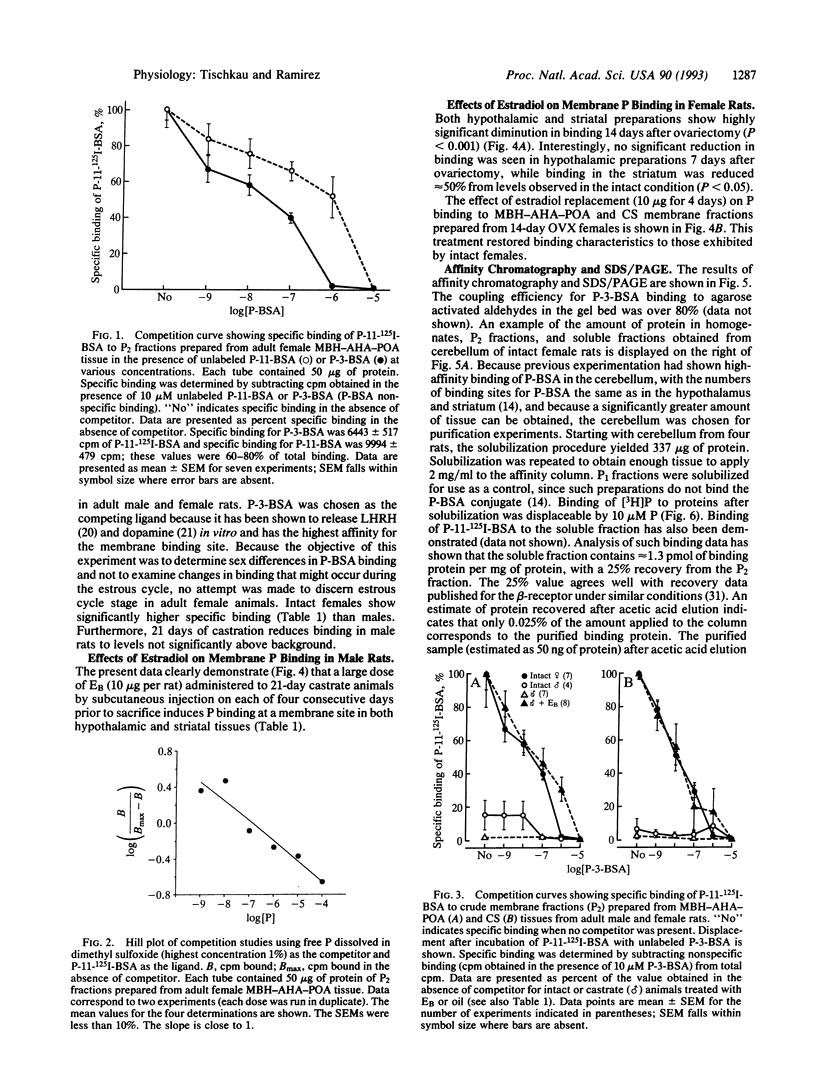
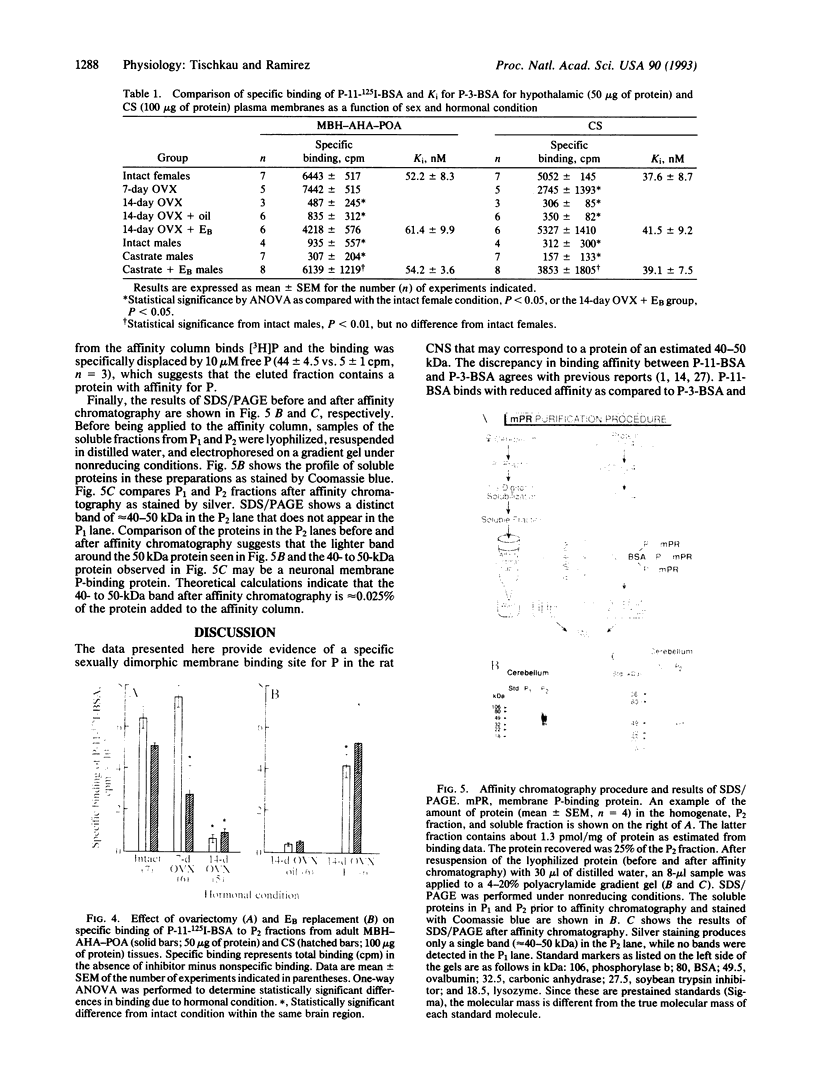
Progesterone conjugated to bovine serum albumin (BSA) was used as a probe to study sex differences and the effects of hormonal status on binding of progesterone to crude synaptosomal membrane preparations (P2) derived from the mediobasal hypothalamic-anterior hypothalamic-preoptic area or the corpus striatum. Binding of 125I-labeled BSA linked to progesterone at the 11 position of the steroid (P-11-BSA) was decreased by competition with unlabeled P-11-BSA or P-3-BSA (in which progesterone is bound to BSA at the 3 position). P-3-BSA displayed higher affinity than P-11-BSA. Hypothalamic and striatal preparations from adult females show high specific binding (60-80%) to the progesterone-BSA conjugate. Specific binding was reduced more than 80% 14 days after ovariectomy. Estrogen treatment (10 micrograms per rat for 4 days) of 14-day ovariectomized rats restored specific binding to levels equivalent to intact females. In contrast, adult males displayed drastically reduced or no specific binding in either tissue. No specific binding was detected after orchidectomy. Estrogen treatment of orchidectomized animals induced specific binding sites similar to those in intact females. Additionally, an affinity probe was developed by linking primary amines on the P-3-BSA conjugate to agarose activated aldehydes in an AminoLink column. A digitoxin-solubilized fraction from female rat P2 cerebellum preparations yielded a single major band after affinity purification with an estimated molecular mass of 40-50 kDa in an SDS/PAGE system after silver stain. These results show a reversible sex difference in the specific binding of progesterone to synaptosomal membrane sites in the central nervous system of male and female rats which is dependent on estrogen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A. P., Gorski R. A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Aronica S. M., Katzenellenbogen B. S. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3',5'-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991 Apr;128(4):2045–2052. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- Barfield R. J., Glaser J. H., Rubin B. S., Etgen A. M. Behavioral effects of progestin in the brain. Psychoneuroendocrinology. 1984;9(3):217–231. doi: 10.1016/0306-4530(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Beebe S. J., Danforth D. R., Alexander N. Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem. 1990 Jan 25;265(3):1376–1380. [PubMed] [Google Scholar]
- Blackmore P. F., Neulen J., Lattanzio F., Beebe S. J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991 Oct 5;266(28):18655–18659. [PubMed] [Google Scholar]
- Bogic L., Gerlach J. L., McEwen B. S. The ontogeny of sex differences in estrogen-induced progesterone receptors in rat brain. Endocrinology. 1988 Jun;122(6):2735–2741. doi: 10.1210/endo-122-6-2735. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown T. J., Clark A. S., MacLusky N. J. Regional sex differences in progestin receptor induction in the rat hypothalamus: effects of various doses of estradiol benzoate. J Neurosci. 1987 Aug;7(8):2529–2536. [PMC free article] [PubMed] [Google Scholar]
- Caron M. G., Lefkowitz R. J. Solubilization and characterization of the beta-adrenergic receptor binding sites of frog erythrocytes. J Biol Chem. 1976 Apr 25;251(8):2374–2384. [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Ramirez V. D. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinology. 1984 Aug;39(2):149–155. doi: 10.1159/000123971. [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Ramirez V. D. In vitro progesterone modulates amphetamine-stimulated dopamine release from the corpus striatum of castrated male rats treated with estrogen. Neuroendocrinology. 1990 Nov;52(5):517–520. doi: 10.1159/000125637. [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Ramirez V. D. Modulatory effects of progesterone upon dopamine release from the corpus striatum of ovariectomized estrogen-treated rats are stereo-specific. Brain Res. 1991 Jan 4;538(1):176–179. doi: 10.1016/0006-8993(91)90395-c. [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Ramirez V. D. Progesterone effects upon dopamine release from the corpus striatum of female rats. II. Evidence for a membrane site of action and the role of albumin. Brain Res. 1989 Jan 9;476(2):338–344. doi: 10.1016/0006-8993(89)91255-9. [DOI] [PubMed] [Google Scholar]
- Duval D., Durant S., Homo-Delarche F. Non-genomic effects of steroids. Interactions of steroid molecules with membrane structures and functions. Biochim Biophys Acta. 1983 Aug 11;737(3-4):409–442. doi: 10.1016/0304-4157(83)90008-4. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., BOREK F., BEISER S. M., LIEBERMAN S. Steroid-protein conjugates. II. Preparation and characterization of conjugates of bovine serum albumin with progesterone, deoxycorticosterone, and estrone. J Biol Chem. 1959 May;234(5):1090–1094. [PubMed] [Google Scholar]
- Feder H. H., Marrone B. L. Progesterone: its role in the central nervous system as a facilitator and inhibitor of sexual behavior and gonadotropin release. Ann N Y Acad Sci. 1977 Mar 11;286:331–354. doi: 10.1111/j.1749-6632.1977.tb29428.x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Ke F. C., Ramirez V. D. Membrane mechanism mediates progesterone stimulatory effect on LHRH release from superfused rat hypothalami in vitro. Neuroendocrinology. 1987 Jun;45(6):514–517. doi: 10.1159/000124784. [DOI] [PubMed] [Google Scholar]
- Majewska M. D. Steroids and brain activity. Essential dialogue between body and mind. Biochem Pharmacol. 1987 Nov 15;36(22):3781–3788. doi: 10.1016/0006-2952(87)90437-0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Orchinik M., Murray T. F., Moore F. L. A corticosteroid receptor in neuronal membranes. Science. 1991 Jun 28;252(5014):1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Rainbow T. C., Parsons B., McEwen B. S. Sex differences in rat brain oestrogen and progestin receptors. Nature. 1982 Dec 16;300(5893):648–649. doi: 10.1038/300648a0. [DOI] [PubMed] [Google Scholar]
- Ramirez V. D., Dluzen D. E., Ke F. C. Effects of progesterone and its metabolites on neuronal membranes. Ciba Found Symp. 1990;153:125–144. doi: 10.1002/9780470513989.ch7. [DOI] [PubMed] [Google Scholar]
- Ramirez V. D., Kim K., Dluzen D. Progesterone action on the LHRH and the nigrostriatal dopamine neuronal systems: in vitro and in vivo studies. Recent Prog Horm Res. 1985;41:421–472. doi: 10.1016/b978-0-12-571141-8.50014-7. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Coirini H., Pfaff D. W., McEwen B. S. Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science. 1990 Nov 2;250(4981):691–694. doi: 10.1126/science.2173139. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Waterhouse B. D., Woodward D. J. Sex steroid effects on extrahypothalamic CNS. II. Progesterone, alone and in combination with estrogen, modulates cerebellar responses to amino acid neurotransmitters. Brain Res. 1987 Sep 29;422(1):52–62. doi: 10.1016/0006-8993(87)90539-7. [DOI] [PubMed] [Google Scholar]
- Su T. P., London E. D., Jaffe J. H. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988 Apr 8;240(4849):219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Valera S., Ballivet M., Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauquelin G., Geynet P., Hanoune J., Strosberg A. D. Isolation of adenylate cyclase-free, beta-adrenergic receptor from turkey erythrocyte membranes by affinity chromatography. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3710–3714. doi: 10.1073/pnas.74.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]