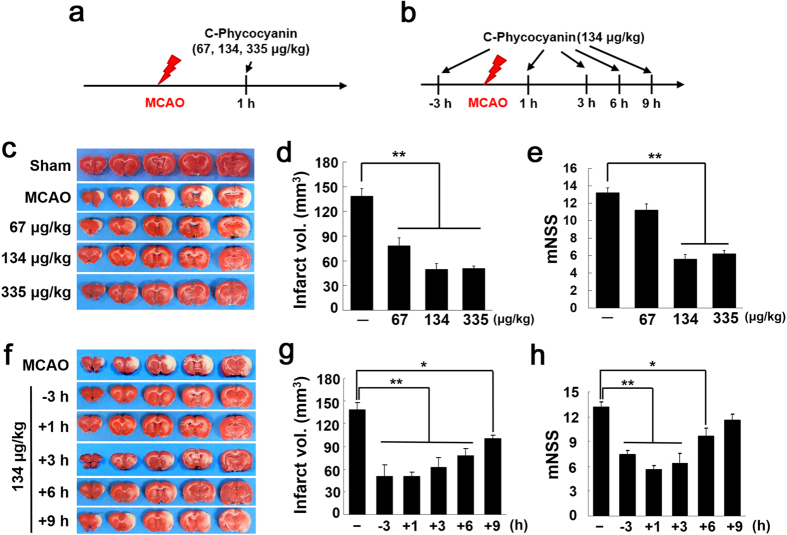
Figure 1. Neuroprotective effects of C-Pc in postischemic brain.
(a,b) MCAO model was prepared and C-Pc was administered intranasally according to the presented schedules. TTC staining and neurological deficits (mNSS) evaluations were conducted 48 h post-MCAO. (c–e) C-Pc (67, 134, or 335 μg/kg) was administered intranasally at 1 h post-MCAO (n = 6–7). (f-h) C-Pc (134 μg/kg) was administered intranasally at 3 h prior to or 1, 3, 6 or 9 h post-MCAO (n = 5–7). (c,f) Representative images of infarctions in coronal brain sections are presented and (d,g) mean infarction volumes were assessed by TTC staining. (e,h) Neurological deficits were evaluated using modified neurological severity scores. Data are presented as the means ± SEMs. The asterisk denotes significant difference against MCAO sample at each concentration and time.*p < 0.05 and **p < 0.01