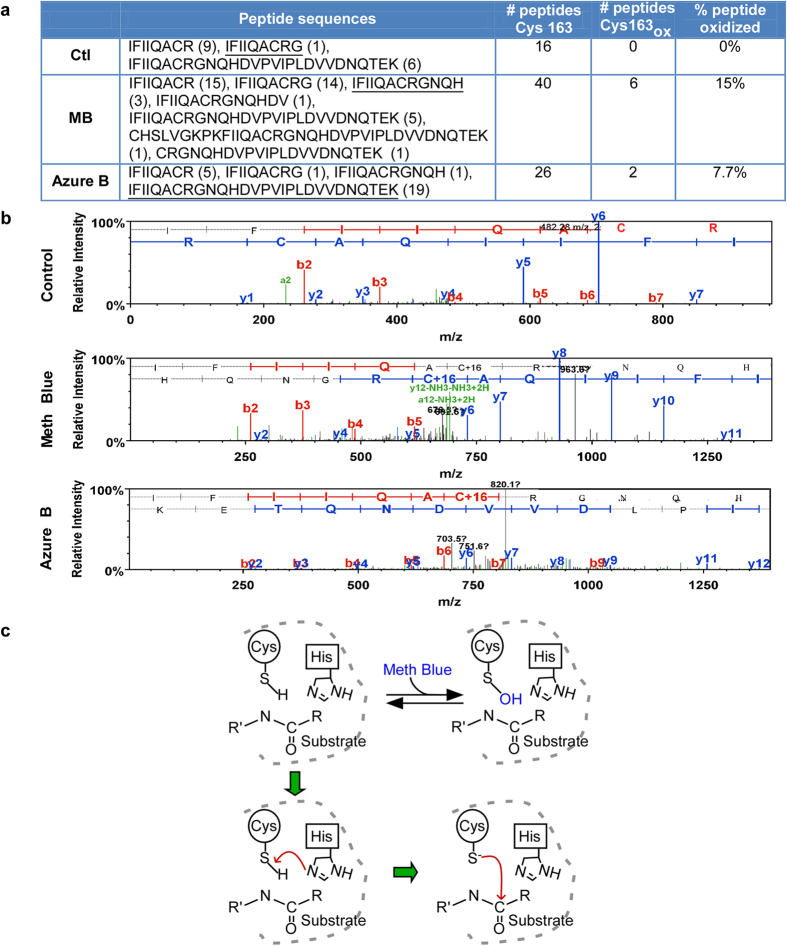
Figure 6. Methylene blue and azure B oxidizes the catalytic Cys163 of active Casp6.
(a) The table represents the number of peptide sequences containing Cys163 obtained by MS/MS and the number of peptides showing Cys163 oxidation. The underlined peptide sequence spectrums are represented in panel b. The percentage of peptides oxidized represents the number of peptides containing an oxidized Cys163 divided by the total number of peptides containing the Cys163 in its sequence. (b) MS/MS spectra showing the oxidized (+16) catalytic Cys163 of Casp6 in the presence of methylene blue or azure B. (c) Schematic representation of Casp6 active site (top left panel). The catalytic mechanism of Casp6 is a multi-step process. The first step (bottom left panel) involves the de-protonation of the active site cysteine thiol by a histidine residue (His 121), thus activating the enzyme. The next step is a nucleophilic attack by the thiolate on the substrate’s peptide carbonyl carbon that subsequently leads to cleavage of the substrate (bottom right panel). However, in presence of methylene blue, the Cys163 thiol group is sulfenated and unable to attack the substrate (top left and right panels).