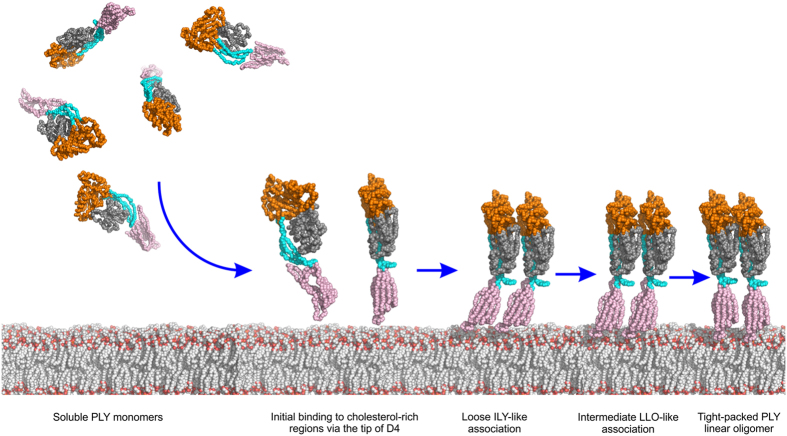
Figure 5. Schematic showing early stages of PLY oligomerisation based on the PLY crystal structure and mapping of the PLY crystal structure on the ILY-CD5919 and LLO26 structures.
PLY is shown as a simplified bead model, coloured by domain as in Fig. 1. PLY approaches the target membrane as soluble monomers and binds to cholesterol-rich regions via the loops at the tip of domain 4. Monomers start to form a loose linear assembly, primarily via domains 1 and 3, as observed in the PLY model based on ILY. As packing between the monomers becomes tighter, the interactions increase to reflect those seen in PLY model based on LLO. Finally, the monomers become tightly packed resembling the linear SDS-sensitive oligomer as seen in the PLY crystal structure. All these linear oligomers are transient in nature but can be trapped by mutation (see text) or in the crystal. In the later stages of cytolytic activity (not shown) interactions with cholesterol trigger conformational changes in domain 3 leading to tilting of the monomers, oligomer circularisation and an SDS-sensitive early prepore. Structural rearrangements in domain 3 lead to the SDS-resistant late prepore before unfurling of the TMH regions in domain 3 to form the SDS-resistant pore (not shown).