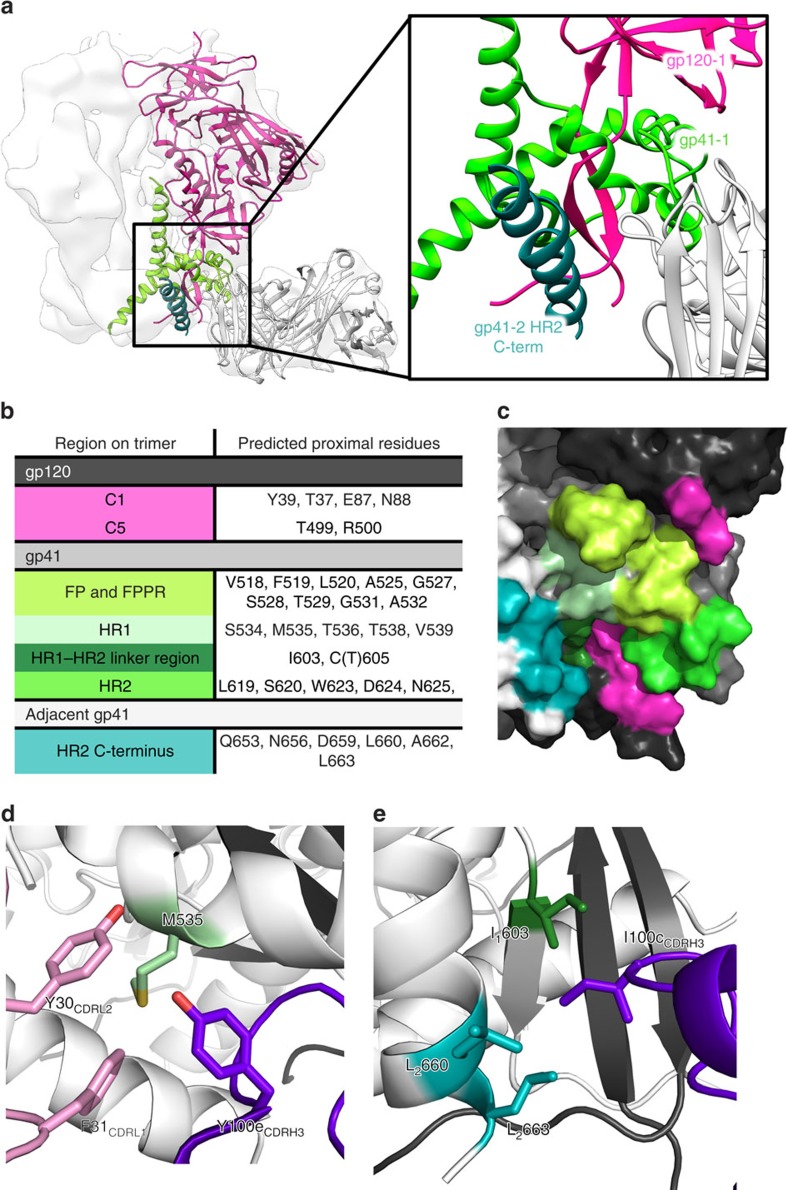
Figure 2. Identification of the 3BC315 epitope by cryo-EM.
(a) On the basis of the docking of crystal structures into the EM reconstruction (white surface), 3BC315 Fab (white cartoon) interacts with gp41 (gp41-1, light green) and gp120 (gp120, pink) within a single gp140 protomer, as well as with the adjacent gp41 (gp41-2, dark green) of the neighbouring protomer. A close up view of this region is shown in the inset. (b) Following RosettaRelax, all side chains in the Env trimer within 5 Å of the antibody CDR loops are shown here. The gp41 residue-forming part of the SOS bond (C605) is also in close proximity. (c) Residues and regions of the trimer that are contacted or buried by the Fab (listed in (b)) are shown on gp120 and gp41 (represented in black and dark grey, respectively) of one protomer, as well as on the adjacent gp41 (white). The 3BC315 epitope is coloured by region as in (b) on the surface rendering of the trimer. (d) M535 in gp41 is surrounded by aromatic residues from CDRH3 (purple) and CDRL1 (pink). (e) I100c from CDRH3 inserts itself into a hydrophobic pocket in the gp41–gp41 interface. Possible interacting residues are labeled and the subscript distinguishes between residues from two gp41 protomers of the trimer.