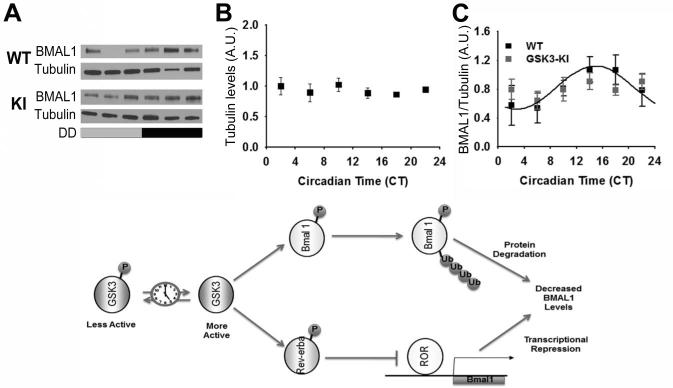
Figure 2. Rhythmic BMAL1 expression in mice with chronic GSK3 activation.
(A) Representative western blots of BMAL1 re-blotted for alpha-tubulin. Quantification (mean ± SEM per CT bin) of (B) alpha-tubulin levels and (C) BMAL1 levels (normalized to alpha-tubulin) when sampled across DD for WT (black; N = 27/time course) and GSK3-KI (gray; N = 25/time course) mice. Cosinor non-linear regression: WT, R2 = 0.23, F(2, 24) = 3.50, p < 0.05; GSK3-KI, R2 = 0.01, F(2, 22) = 0.57, p = 0.91. (Bottom) Schematic representing hypothetical model for the interrelationship between GSK3 and the circadian clock. Circadian clock dependent regulation of GSK3 results in time-ofday-dependent oscillations in target proteins, including BMAL1 and REV-ERBα. Phosphorylation of BMAL1 primes this clock component for ubiquitination and subsequent degradation, while phosphorylation of REV-ERBα promotes nuclear translocation and inhibition of ROR-mediated Bmal1 transcription. Increased BMAL1 protein degradation coupled with diminished Bmal1 transcription will result in diminished BMAL1 protein levels and therefore attenuated circadian clock output.