Scheme 4. Synthetic route to ABC diepoxide-opening cascade precursor and formal synthesis target.
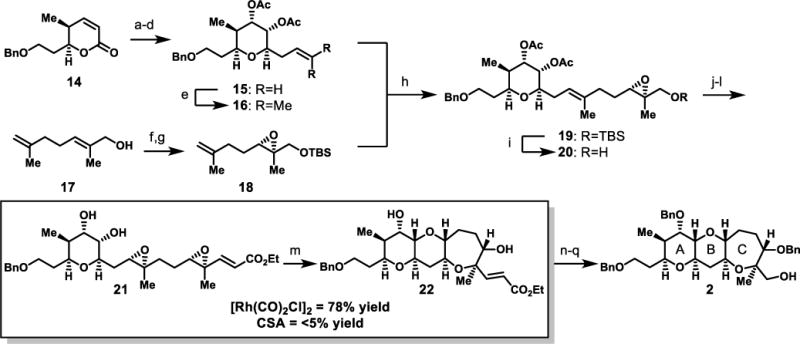
a) OsO4, NMO, Acetone/H2O (4:1), 95%; b) CH2=CHCH2MgBr, THF, −78 °C; c) Et3SiH, TMSOTf, MeCN, 44% (over 2 steps); d) Ac2O, pyr, DMAP, CH2Cl2, 87%; e) 2-methyl-2-butene, Hoveyda-Grubbs cat. (2nd generation), Benzoquinone, 87%; f) L-(+)-DET, Ti(OiPr)4, TBHP, CH2Cl2, −20 °C, 49%, 93% e.e.; g) TBSCl, Et3N, CH2Cl2, 86%; h) Hoveyda-Grubbs cat. (2nd generation), Benzoquinone, 80 °C, 78%, 2:1 E/Z; i) TBAF, THF, 84%; j) pyr•SO3, DMSO, Et3N, CH2Cl2, rt; then Ph3PCHCO2Et, rt, 91%; k) (+)-12, KHSO5, Bu4NHSO4, K2CO3, pH 10.5, DMM/CH3CN (2:1), 84%, 3:1 d.r.; l) Guanidine•HCl, NaOEt, EtOH, 77%; m) [Rh(CO)2Cl]2 (10 mol%), THF, rt, 78%; n) NaH, BnBr, TBAI, THF, 60 °C, 75%; o) K2OsO2(OH)4, Citric Acid, NMO, t-BuOH/H2O; p) Ph3BiCO3, CH2Cl2, reflux; q) NaBH4, MeOH, 0 °C, 60% (over 3 steps).
