Table 2.
Trisubstituted epoxy alcohol cyclizations under [Rh(CO)2Cl]2 and (±)-CSA promotion
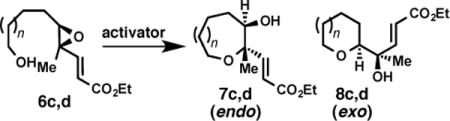
| ||||
|---|---|---|---|---|
| entry | substrate | activator | 7/8 | yield 7 (%)a |
| 1 | 6c, n = 0 | [Rh(CO)2Cl]2b | >20: 1 | 93 |
| 2 | 6c, n = 0 | (±)-CSAc | 3.4: 1 | – |
| 3 | 6d, n = 1 | [Rh(CO)2Cl]2d | >20: 1 | 88 |
| 4 | 6d, n = 1 | (±)-CSAe | 1: 1.8 | – |
Isolated yield.
[Rh(CO)2Cl]2 (1 mol %), THF, rt.
(±)-CSA (10 mol %), CH2Cl2, rt.
[Rh(CO)2Cl]2 (2.5 mol %), THF, rt.
(±)-CSA (100 mol %), CH2Cl2, rt.
