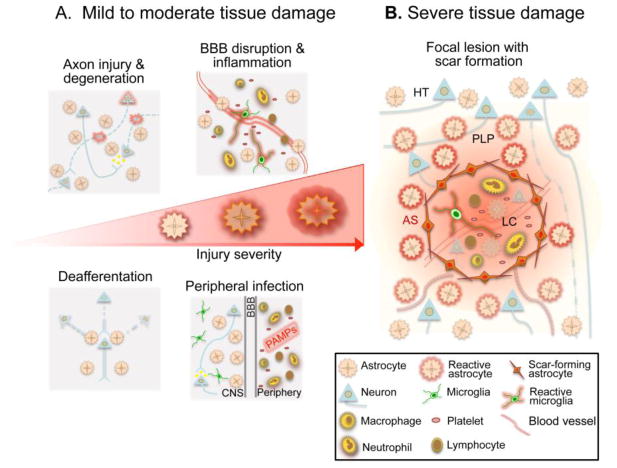
Figure 1. Reactive astrogliosis following TBI is a graded and heterogeneous response that reflects the severity of CNS tissue damage.
A, In response to mild or moderate tissue damage, astrocytes undergo hypertrophic reactive astrogliosis that includes molecular, structural and functional changes. Different forms of tissue pathology, such as local axonal injury and degeneration, blood brain barrier (BBB) disruption with inflammatory cell extravasation, deafferentation and synapse degeneration due to distal axon injury, or exposure to PAMPs associated with peripheral bacterial or viral infection, can all uniquely influence astrocyte function and in different combinations can drive specific forms of astrogliosis. These hypertrophic reactive astrocytes are intermingled among viable neural cells in areas of injured, but surviving and functioning neural tissue. B, Severe tissue damage elicits neural and glial cell degeneration, vascular breakdown and a robust innate and adaptive immune response, leading to the formation of tissue compartments with distinct forms of reactive astrogliosis. Immediately adjacent to the injury, astrocytes proliferate and intertwine to form an astroglial scar (AS) that surrounds and restricts the spread of the intense inflammatory response in the lesion core. These scar forming astrocytes are present in areas that contain few if any surviving neural cells, and their main interactions are with non-neural cells in tissue lesions. Adjacent to the astrocyte scar, features characteristic of mild or moderate brain trauma are present and taper with distance from the lesion core (LC). In these areas reactive astrocytes undergo changes in morphology and function characteristic of hypertrophic reactive astrogliosis as described in A, and these reactive astrocytes interact with injured but surviving cells in the perilesion perimeter (PLP). Astrocyte reactivity in the PLP may also influence neurons and glia in the healthy tissue (HT) distal to the injury.