Abstract
The neuroprotective effects of progesterone after ischemic stroke have been established, but the role of progesterone in promoting cerebrovascular repair remains under-explored. Male Sprague–Dawley rats underwent transient middle cerebral artery occlusion (tMCAO) for 90 min followed by reperfusion for 3 days. Progesterone (8 mg/kg/day) was administered intraperitoneally at 1 hour after initial occlusion followed by subcutaneous injections at 6, 24 and 48 hours post-occlusion. Rats were euthanized after 72 hours and brain endothelial cell density and macrophage infiltration were evaluated within the cerebral cortex. We also assessed progesterone’s ability to induce macrophage migration towards hypoxic/reoxygenated cultured endothelial cells. We found that progesterone treatment post-tMCAO protects ischemic endothelial cells from macrophage infiltration. We further demonstrate that infiltration of monocytes/macrophages can be induced by potent chemotactic factors such as monocyte chemoattractant protein-1 (MCP-1) and the chemokine ligand 1 (CXCL1), secreted by hypoxic/reoxygenated endothelial cells. Progesterone blunts secretion of MCP-1 and CXCL1 from endothelial cells after hypoxia/reoxygenation injury and decreases leukocyte infiltration. The treatment protects ischemic endothelial cells from macrophage infiltration and thus preserves vascularization after ischemic injury.
Keywords: brain ischemia, cerebrovascular repair, endothelial cells, macrophage infiltration, progesterone
Introduction
Our laboratory and others have demonstrated that in many pre-clinical studies progesterone has substantial neuroprotective effects against brain injury, including traumatic brain injury (TBI) and ischemic stroke (Sayeed et al., 2007; Ishrat et al., 2009; Kaore et al., 2012; Won et al., 2014). Progesterone has been shown to reduce infarct volume, edema, global inflammation and behavioral deficits in both permanent and transient rodent models of middle cerebral artery occlusion (MCAO) compared to vehicle-injected controls (Sayeed et al., 2007; Gibson et al., 2009; Ishrat et al., 2009; Jiang et al., 2009; Ishrat et al., 2012; Kaore et al., 2012). As previously reported, progesterone significantly reduces infarct volume 72 hours after transient (t)MCAO (Sayeed et al., 2006). Although we are learning more about the role of progesterone in neuroprotection and repair, many of the molecular mechanisms and specific cell type(s) mediating its beneficial effects after ischemic/reperfusion injury remain to be determined. One of the mechanisms that can reduce infarct size after ischemia may be increased blood flow, which in turn leads to neurovascular remodeling processes and functional recovery.
After ischemic/reperfusion injury, increased vascularization or increased vascular protection may be critical to mediate functional recovery, with endothelial cells being the primary effector cell type responsible for neo-vascularization and angiogenesis (Sunderkotter et al., 1994; Folkman, 1995; Cao et al., 2005). Functional recovery of ischemic tissues and organs is dependent on vascular networks that sufficiently supply hyper-oxygenated blood to endothelial cell populations. Therefore, to reduce the severity of the injury, preserving endothelial cell viability and function is of utmost importance after ischemic/reperfusion injury. As a component of the post-stroke injury cascade, macrophage infiltration can trigger apoptosis of healthy cells in vivo, including endothelial cells (Diez-Roux and Lang, 1997). Conversely, macrophage infiltration can also promote wound healing and scar tissue formation after ischemic injury, and facilitate recovery (Frangogiannis, 2004). We are interested in understanding endothelial cell viability and macrophage recruitment patterns post-injury, in both the presence and absence of progesterone. It is plausible that progesterone could facilitate communications between ischemic endothelial cells and peripheral macrophages. Macrophages can be recruited to ischemic tissue by cytokines and chemokines that are expressed by endothelial cells (Stroo et al., 2010). Depending on tissue type, whether macrophage infiltration and/or chemokine secretion facilitate injury progression or prevent further damage after ischemic reperfusion injury remains highly controversial (Lambert et al., 2008; Akcay et al., 2009; Nahrendorf et al., 2010; Stroo et al., 2010).
Monocyte chemoattractant protein, also known as MCP-1/CCL2, is a critical chemokine involved in the positive chemotaxis of macrophages towards endothelial cells after both kidney and myocardial ischemic reperfusion injury (Shyy et al., 1994; Stroo et al., 2010). The murine chemokine keratinocyte chemoattractant CXCL1/KC, a potent neutrophil chemoattractant, is also heavily involved in ischemic/reperfusion injury (Shea-Donohue et al., 2008). Because tissue damage and repair are dependent on the timely induction and suppression of chemokines, it is important to identify what modulates chemokine expression during ischemic injury.
Because of progesterone’s long history of demonstrating neuroprotection after CNS injury, the purpose of this study was to test its effects on vascularization and/or cerebral vascular protection after ischemic injury. We evaluated progesterone-mediated MCP-1- and CXCL1-dependent macrophage infiltration and its effects on the cerebral vasculature following ischemic injury.
Here we demonstrate that an important mechanism by which progesterone decreases the severity of ischemic/reperfusion injury is by decreasing macrophage infiltration and thus protecting cerebrovascular endothelial cells from inflammation.
Methods
Subjects
Adult male Sprague Dawley rats (250–350 g; Charles River Laboratories, Wilmington, MA) were used according to procedures approved by the Institutional Animal Care and Use Committee, Emory University, Atlanta GA (protocol # DAR- 2001411). All animals were housed in an AAALAC-approved Research Animal Facility with a temperature-, humidity-, and light-controlled environment, and placed under a 12-hour reverse light–dark cycle. Public Health Service Policy on Humane Care and Use of Laboratory Animals, the Guide for the Care and Use of Laboratory Animals, and all other applicable regulations, policies, and procedures were followed and approved by Emory University Institutional Animal Use and Care Committee. The experiments are reported here in accordance with the ARRIVE guidelines. Animals were randomized into three groups (n = 6–8 each): sham-operated vehicle-treated control; tMCAO + vehicle; and tMCAO + progesterone (8 mg/kg). The rats were assigned randomly to the treatment groups and group identity was coded with regard to surgery and treatment.
Drug preparation
Reagent grade progesterone (P-3972; Sigma Aldrich Co., St Louis, MO) was dissolved in 22.5% 2-hydroxypropyl-β cyclodextrin and administered by intraperitoneal (IP) injection 1 h post-occlusion to ensure rapid delivery (Ishrat et al., 2010), and then subcutaneously (SC) at 6, 24, and 48 h post-occlusion. The 8 mg/kg dose is based on previous dose response studies in rodents demonstrating that this amount provides maximal protective benefits in the treatment of various types of brain injury (Ishrat et al., 2009; Wali et al., 2014; Yousuf et al., 2014).
Transient Middle Cerebral Artery Occlusion Injury Model
Focal cerebral ischemia was induced by occlusion of the right middle cerebral artery as previously described (Longa et al., 1989). Briefly, a midline incision was made on the ventral surface of the neck and the right common carotid artery was isolated and ligated with a 6.0 silk suture. The internal carotid artery and the pterygopalatine artery were temporarily occluded using a microvascular clip. A 4–0 nylon monofilament with a heat-blunted tip was introduced into the internal carotid artery through the incision in the external carotid artery. Relative cerebral blood flow was monitored for the entire 90 min of occlusion using laser Doppler (LD) perfusion. Only rats with a mean ischemic cerebral blood flow less than 40% of baseline LDF were included to reduce variability and ensure relative uniformity of the ischemic insult. Mean relative cerebral blood flow observed during occlusion in rats treated with progesterone (26.26%±4.03%) was not statistically different from that observed in vehicle-treated animals (25.69%±3.71%). Reperfusion, as demonstrated by early recovery of the LD flowmetry signal, occurred in all animals regardless of treatment group within minutes of occluding filament release. There were no significant differences in the increase in mean relative cerebral blood flow in the progesterone-treated (93.47%±9.36%) animals compared with the vehicle-treated group (101.80%±11.17%).
At 5 min before the onset of reperfusion, drug treatment was administered by IP injection. After 90 min of MCAO, the occluding filament was withdrawn from the common carotid artery to allow reperfusion. One animal died during the tMCAO procedure (prior to treatment) for reasons unknown and was not included in further analyses.
Histology and immunohistochemistry
Microvessel density, endothelial cell proliferation and macrophage density in the penumbra cortex were detected by immunohistochemistry, using previously reported methods as a guideline (Wang et al., 2012). Transcardial perfusion was performed and brains were fixed with 10% formalin, harvested and then dehydrated in a 15%, 20% and 30% serial sucrose gradient. Brains were then snap-frozen and cryo-cut coronally at 30 μm. Brain sections were incubated overnight at 4° C with the following primary antibodies: rat blood-brain barrier (SMI 71) monoclonal antibody (1:1000, Covance, Princeton, NJ) to detect endothelial cells; CD68 (1:200, Abcam, Cambridge, MA) to detect tissue macrophages; Ki67 (1:500, Abcam) to detect cell proliferation; and vascular endothelial growth factor (VEGF, Santa Cruz, Santa Cruz, CA) to determine the pro-angiogenic potential of endothelial cells.
For SMI71, CD68, VEGF and Ki67, sections were incubated with the appropriate fluorescence dye-conjugated secondary antibodies (1:200, Invitrogen, Carlsbad, CA). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) included in the mounting medium (Vector Laboratories, Burlingame, CA). Immunolabeling signals were captured using a Zeiss fluorescent microscope and quantified using a 20X objective lens from 5 random fields from each section and from 3 independent sections for each brain.
Cell culture assays and hypoxic injury model
Commercially available endothelial cells (bEnd.3, ATCC, Manassas, VA) derived from the cerebral cortex of mice were used for oxygen glucose deprivation (OGD) studies as previously reported (Hiu et al., 2008). Cells were grown in high-glucose Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen) in the presence of 10% fetal bovine serum (ATCC) and penicillin/streptomycin. For normoxic conditions (>20% oxygen), the cells were transferred into serum-free media containing 4.5 g/l glucose (control media). To induce hypoxia, cells were transferred to a serum- and glucose-free DMEM media that was pre-bubbled with nitrogen gas for 30 min. Hypoxia conditions consisted of 2 h hypoxia in a standard hypoxia chamber set to 0.1% oxygen, with or without the administration of 20 μM progesterone. Reoxygenation was initiated by adding serum-free, normoxic media (Hiu et al., 2008). TIB-186/IC21 cells were graciously donated by Robert Taylor, Emory University, and cultured in RPMI (Gibco, Grand Island, NY) containing 15% fetal bovine serum (ATCC) and penicillin/streptomycin. Non-treated and non-hypoxic controls were also included within this assay.
Two Chamber Trans-well Migration Assay
An 8-μm polycarbonate membrane trans-well assay (Costar Corning, Tewksbury, MA) was used to determine the migratory potential of macrophages towards hypoxia stimulated endothelial cells (Reinhart-King, 2008). bEnd.3 endothelial cells were seeded at a density of 1 × 105 cells/well in complete DMEM and cultured to confluence into the lower chamber of a 24-well plate. The endothelial cells were then subjected to hypoxia for 2 h and reperfusion for 4 h (+/− progesterone). During reoxygenation, cultured macrophages (TIB-186/IC21, ATCC) were seeded at a density of 1 × 104 in the upper trans-well chamber and allowed to migrate during the reperfusion stage for 4 h. After 4 h, macrophages were fixed and stained with DAPI. Cells from 4 random fields per porous filter were quantified for each condition.
Cytokine-specific proteome array
Cytokine release into conditioned media after hypoxia/reperfusion +/− progesterone treatment was measured utilizing a Mouse Cytokine Array kit (ARY006) (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Briefly, conditioned media (8 ml) from bEnd.3 cells treated with progesterone (20 μM) for 2 h during hypoxia and 4 h during reperfusion were concentrated 3-fold using a Pierce Concentrator. After blocking, array panels were incubated in a mixture of conditioned media (1.5 ml) and a cocktail of biotinylated detection antibodies (15 μl) overnight at 4° C. Any cytokine/detection antibody complex present is bound by its cognate immobilized capture antibody on the membrane. After a wash to remove unbound material, streptavidin-horseradish peroxidase and chemiluminescent detection reagents were added sequentially. To validate results obtained by the proteome profiler, concentrations of MCP-1/CCL2 and Cxcl1 chemokines were measured by ELISA (R&D Systems) using manufacturer’s recommendations. Experiments were performed 6 independent times.
Protein extraction and Western blotting
Thirty μg of protein lysed in radio-immunoprecipitation assay buffer (RIPA) were loaded onto 7.5% SDS polyacrylamide gels (BioRad, Hercules, CA) and subsequently transferred to PDVF membranes (Amersham, Piscataway, NJ). Membranes were blocked with 5% milk. The TNF-α antibody (Abcam) was used at a 1:5000 dilution. Blots were analyzed using densitometry software (Un-Scan-It, Orem, UT) and normalized to β-actin (Cell Signaling, Danvers MA), used at a 1:10,000 dilution.
Statistical analysis
Based on a delta-value of 1.25, we calculated the sample sizes and power needed to reject the null hypothesis to achieve 80% power to detect a 50% difference in the outcome measures indicated above. The number of rats per group at these criteria was determined to be at least six to reject the null hypothesis (H0) at the 0.05 level at a power of 0.8. The parameters were analyzed using one-way analysis (ANOVA, 2 tailed) of variance followed by the Bonferonni post hoc test. All data are presented as mean±s.e.m. All tests were considered statistically significant at p values less than 0.05.
Results
Progesterone protects endothelial cell population 3 days after tMCAO
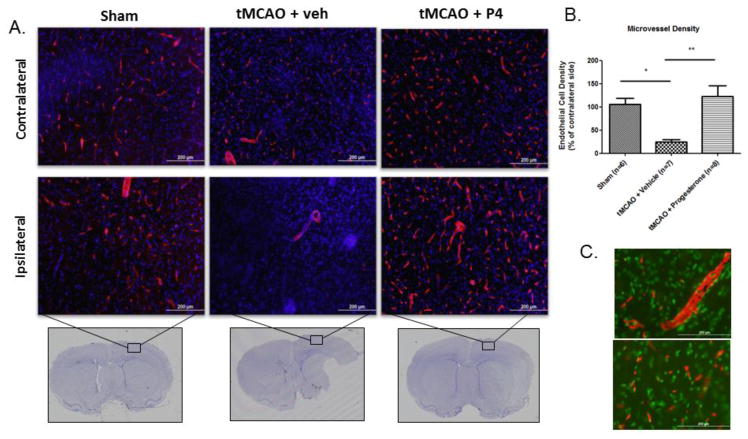
The literature suggests angiogenesis increases as early as 7 days post-tMCAO surgery within the cerebral cortex and is maintained over 14 days. This process is quantified by measuring increases in endothelial cell density compared to sham controls (Wang et al., 2012). Since we have shown that progesterone administration improves functional recovery as early as 3 days after tMCAO, we monitored angiogenesis at 3 days to determine whether there were increases in this parameter after progesterone treatment (Sayeed et al., 2006). After tMCAO surgery, 3 days of progesterone treatment significantly increased endothelial cell density on both the injured and the intact sides of the brain compared to vehicle controls (Figure 1A). We also observed that the ipsilateral cortex of tMCAO animals given vehicle showed little to no endothelial cell population within the cerebral cortex (Figure 1A). The injury produced extensive loss of ipsilateral endothelial cells 3 days following ischemia/reperfusion injury (Figure 1B), which was attenuated by progesterone treatment (Figure 1B; p < .05). We evaluated endothelial cell density with a second endothelial marker, EBA, and were able to confirm these results (Supplementary Figure 1). We also evaluated angiogenesis 8 days post-tMCAO and found that tMCAO itself increases ipsilateral endothelial cell density compared to sham controls when compared to cell density seen in the contralateral hemisphere (p < .05; data not shown). To verify whether the progesterone cerebral vascular effect was protective or proliferative, we performed immunostaining with proliferation markers VEGF or Ki67. Endothelial cells from cerebral cortical sections of progesterone-treated rodents did not co-stain with the proliferation markers VEGF or Ki67 (Figure 1C). An in vitro test of hypoxic endothelial cells undergoing reoxygenation in the presence of progesterone also failed to show increased VEGF expression or an increase in cell numbers (data not shown). Thus far, our results can be taken to suggest that progesterone does, in fact, protect preexisting cerebral vascular endothelial cells and further demonstrates that this population of endothelial cells is not the result of progesterone-mediated proliferation/angiogenesis.
Figure 1.
Progesterone protects endothelial cell population 3 days post-tMCAO. (A) Representative images of staining for endothelial cells using blood-brain barrier (SMI 71) from the cerebral cortex 3 days post-tMCAO and progesterone (P4)/vehicle (veh) treatment. Top panel shows endothelial cell (red) density in the contralateral (control) region of the ischemic brain while the lower panel shows endothelial cell density on the ipsilateral ischemic side of the cerebral cortex (box on Cresyl violet stained section shows region of interest for immunostaining). (B) Endothelial cell density normalized to contralateral hemisphere. tMCAO reduced endothelial cell density 3 days post-injury compared to sham controls, while progesterone significantly increased endothelial cell density over vehicle controls. (C) Representative images from the cerebral cortex of progesterone-treated animals analyzed for angiogenesis. VEGF (red) does not co-localize with endothelial cells (green). *p <0 .05, **p<0.001; n = sham (6), tMCAO + veh (7), P4 (8)
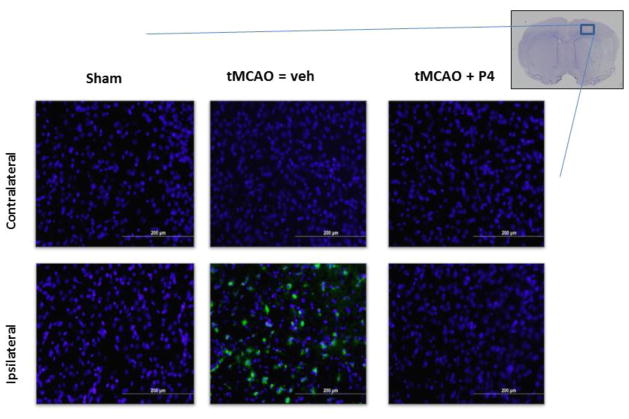
Progesterone blunts macrophage infiltration 3 days post-tMCAO
Significant numbers of macrophages were present within the injured/ipsilateral cerebral cortex of vehicle-treated animals, but more importantly, these cells were not present in the progesterone-treated tMCAO group (Figure 2). Other than sections from tMCAO animals treated with vehicle, no positive staining for macrophages, as measured by ED-1/CD68, was observed in any of the other cerebral cortical sections (Figure 2). We obtained a significant decrease in endothelial cell density (Figure 1) with a concomitant increase in macrophage infiltration in response to ischemia/reperfusion injury (Figure 2), as seen in vehicle control animals.
Figure 2.
Progesterone blunts macrophage infiltration. (A) Representative images of staining for macrophages using ED1/CD68 in the cerebral cortex 3 days post-tMCAO. Top panel shows contralateral hemisphere where there are no macrophages in any of the treatment groups. Lower panel shows macrophages present only in the vehicle-treated tMCAO group. n = sham (3), tMCAO + veh (7), progesterone (P4) (3).
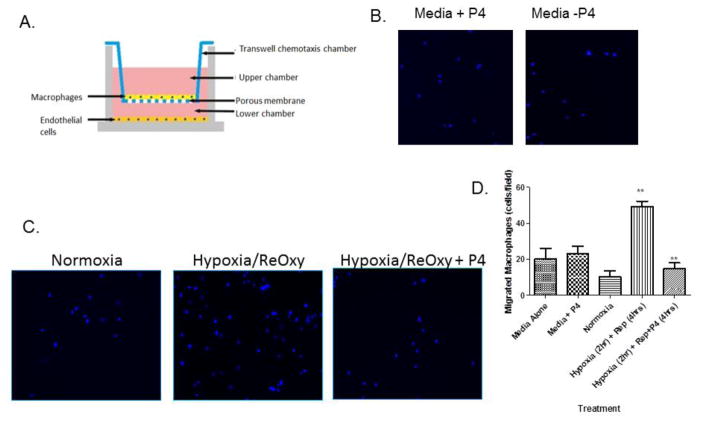
Progesterone inhibits macrophage migration towards hypoxic endothelial cells
During normoxic conditions, a basal number of macrophages migrated toward endothelial cells. Endothelial cells subjected to hypoxia/reoxygenation resulted in a 5-fold increase in macrophage migration, which was blunted by the addition of progesterone (Figure 3C; p < .05). We observed that media treated with progesterone alone is not responsible for the difference in macrophage migration patterns, as evidenced by the lack of change in macrophage migration (Figure 3B). Indeed, progesterone has an effect on macrophage migration only when endothelial cells are present, suggesting that the hormone functions to modulate endothelial cell factor secretion, which in turn alters macrophage recruitment after hypoxia/reperfusion injury. Our finding is in line with our in vivo staining of the cerebral cortex, which shows the presence of macrophages after tMCAO with vehicle treatment, and no macrophage response following progesterone given after tMCAO (Figure 2). We hypothesize that endothelial cells exposed to hypoxic/ischemic conditions secrete factors that influence macrophage recruitment and that the factors secreted are modulated by progesterone treatment.
Figure 3.
Progesterone-treated hypoxic endothelial cells inhibit macrophage migration. (A) Schematic of the two-chamber two-cell transwell assay. Endothelial cells (bEnd.3) subjected to hypoxia/reoxygenation are plated in the bottom chamber and macrophages (IC21) are suspended in the top chamber and allowed to migrate. Representative 20x images of macrophages (stained by DAPI) after migrating toward (B) media alone +/− progesterone or (C) hypoxic endothelial cells +/− progesterone. (D) Hypoxia/reoxygenation increased macrophage migration compared to normoxia controls and was blunted by the addition of progesterone. (***p <0 .0001; n = 4)
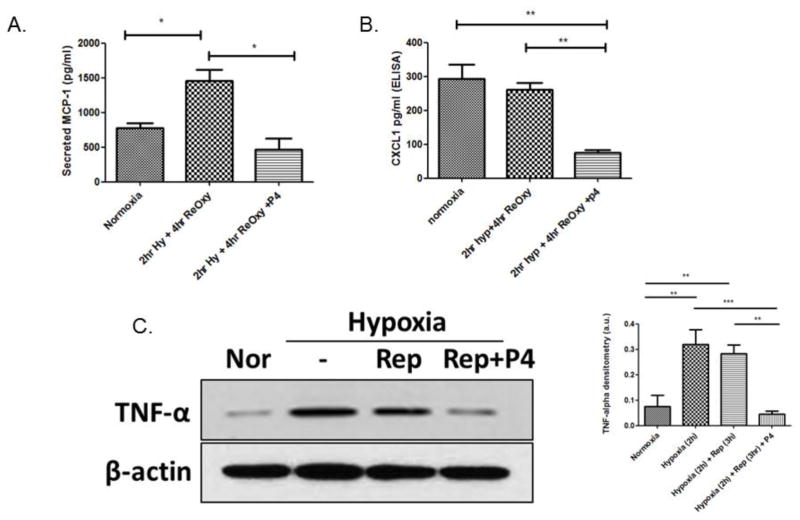
Progesterone blunts chemokine expression
Of 32 candidate chemokines and cytokines, we identified four that were modulated after exposure to hypoxia/reperfusion and progesterone: MCP-1/CCL2, CXCL1/KC, tissue inhibitor of metalloproteinases (TIMP1) and chemokine ligand 2 (MIP-2/CXCL2). Based on literature searches, two of the candidates, MCP-1/CCL2 and CXCL1/KC, were previously associated with ischemia-mediated macrophage infiltration. Further validation of these two candidates of interest was performed using specific ELISAs. We found that expression of both MCP-1/CCL2 and CXCL1/KC, which typically is elevated after ischemic injury (Stroo et al., 2010), was inhibited after exposure to progesterone (Figure 4A and B; p < .05)). It is noteworthy that CXCL1/KC secretion trended towards increased expression from normoxia to hypoxia/reoxygenation injury, suggesting that this chemokine may be maintained at basal levels, except when in the presence of progesterone, when its secretion is blunted (Figure 4B; p < .05) Our results show that chemokines are involved in recruiting macrophages into the endothelial space post-infarction and that progesterone can blunt the recruitment of macrophages, thus prolonging and protecting the stability of preexisting endothelial cells.
Figure 4.
Progesterone blunts inflammatory cytokine secretion from hypoxic endothelial cells. (A) MCP-1/CCL2 release after hypoxia/reoxygenation. (B) CXCL1/KC release after hypoxia reoxygenation. Cytokine release is significantly increased compared to normoxia after hypoxia/reoxygenation and significantly decreased after the addition of progesterone. p < .05 n = 6 (C) Cellular TNF-α expression after hypoxia/reoxygenation, blunted after the addition of progesterone.
It is well documented that pro-inflammatory cytokines such as TNF-α and IL-1β stimulate chemokine synthesis in ischemic tissues (Frangogiannis, 2004). Accordingly, we evaluated the expression of TNF-α in our hypoxic endothelial cells and found, as expected, that hypoxia and hypoxia/reperfusion increase TNF-α. We also found that progesterone blunts TNF-α expression to levels similar to normoxia levels, and TNF-α may be a key signaling molecule leading to the secretion of MCP-1 and CXCL1 chemokines (Figure 4C).
Discussion
Here we propose a novel mechanism of action by which progesterone functions to protect endothelial cells from macrophage-mediated infiltration, inflammation and death. The basis for this protection is related to chemokine expression patterns of hypoxic endothelial cells, which can initiate the recruitment or suppression of macrophages into the zone of injury. This macrophage/chemokine effect is conserved within in vivo and in vitro models of ischemic stroke. Indeed, progesterone has an effect on macrophage migration only when endothelial cells are present, suggesting that the hormone functions to modulate endothelial cell factor secretion, which in turn alters macrophage recruitment after hypoxia/reperfusion injury. Our finding is in line with our in vivo staining of the cerebral cortex, which shows the presence of macrophages after tMCAO with vehicle treatment, and no macrophage response following progesterone given after tMCAO (Figure 2). We hypothesize that endothelial cells exposed to hypoxic/ischemic conditions secrete factors that influence macrophage recruitment and that the factors secreted are modulated by progesterone treatment.
In the present study, we found that endothelial cells are protected in the cerebral cortex by progesterone within 3 days after ischemic/reperfusion injury induced by tMCAO. We also noted that without progesterone, the endothelial cell population in the area of the infarct was eliminated and replaced by a significant presence of macrophages in the cerebral cortex. We were unable to identify a relationship between these progesterone-protected endothelial cells and angiogenic and/or arteriogenic markers, leading us to conclude that progesterone was in fact protecting preexisting endothelial cells from the macrophage-induced cytotoxicity post-ischemic/reperfusion injury rather than stimulating the angiogenesis that follows the injury. Based on the results of our experiment we think that macrophage infiltration is detrimental to the promotion of perfusion recovery after ischemic injury. This finding, taken together with the fact the endothelial cells from the progesterone group were negative for angiogenic markers (Figure 1C), leads us to hypothesize that the preexisting population of endothelial cells is protected from macrophage infiltration and, ultimately, macrophage-induced death.
In our in vitro experiment we found that progesterone blunted the migration of macrophages toward hypoxia/reoxygenated endothelial cells. Interestingly, progesterone-treated media in the absence of injured cells was not sufficient to induce macrophage migration, because progesterone is not in itself a migratory stimulus for macrophages. Our study shows that the hypoxic/reoxygenated condition of the endothelial cell is necessary to stimulate macrophage migration patterns. By profiling the media from hypoxic/reoxygenated endothelial cells, we identified candidate chemokines modulated by progesterone treatment. Two chemokines involved in macrophage recruitment were reduced in the presence of progesterone: MCP-1/CCl2 and CXCL1/KC.
Because Farivar, et al. have previously shown that TNF-α-deficient mice undergoing experimental infarction exhibit decreased chemokine expression, this suggested an important role for TNF-α in mediating the post-infarction chemokine response (Farivar et al., 2004). Other studies have linked TNF-α and chemokine expression in the rat myocardium, where lipopolysaccharide-induced CXC chemokine (LIX) is expressed by resident myocardial cells during ischemia-reperfusion and is induced in cultured cardiomyocytes by TNF-α via NF-κB activation (Chandrasekar et al., 2001). Based on these reports, we evaluated the expression of TNF-α in our cell culture model after hypoxia/reoxygenation and found that, in line with current literature, progesterone decreases TNF-α expression to modify the injury cascade.
The influence of chemokines on ischemic reperfusion injury is very much time-dependent and tissue-specific. Previous studies have identified a role for chemokines in renal protection after ischemic injury but none have implicated progesterone as a regulator. Stroo et al. demonstrated that during renal ischemic/reperfusion injury, tubular epithelial cells produce chemokines that upon inhibition and/or neutralization ameliorate the initiation and progression of renal damage (Stroo et al., 2010). Sung et al. showed a similar result, suggesting that not only is MCP-1-dependent macrophage infiltration contributory to renal ischemic damage, but also that inhibition reduced the severity of injury (Sung et al., 2002). In experimental diabetic nephropathy, Ccl2/MCP-1 deficiency decreased renal damage by reducing the accumulation of macrophages. Inhibition of Ccl2/MCP-1 signaling and neutralizing Cxcl1/KC during the inflammatory response has a protective effect on ischemic renal injury and also attenuates hepatocellular injury after ischemia/reperfusion (Shea-Donohue et al., 2008).
In contrast to reports implicating chemokines in the progression of ischemic renal injury, some myocardial studies have linked increased chemokine expression with beneficial effects after reperfusion injury (Morimoto et al., 2008). Myocardial infarction depends on the infiltration of macrophages to promote scar tissue, neovascularization, remodeling and wound healing (Lambert et al., 2008; Morimoto et al., 2008; Nahrendorf et al., 2010). We now have shown that cerebrovascular endothelial cells appear to function in ways similar to the kidney in response to chemokine secretion and macrophage infiltration after ischemic reperfusion injury in the brain. We propose that elevated chemokine expression (particularly MCP-1 and CXCL1) contributes to cerebral ischemia/reperfusion injury and that progesterone blunts the secretion of these factors by ischemic endothelial cells (Figure 5). This process will then lead to reduced macrophage infiltration, more vascular protection and functional recovery after stroke.
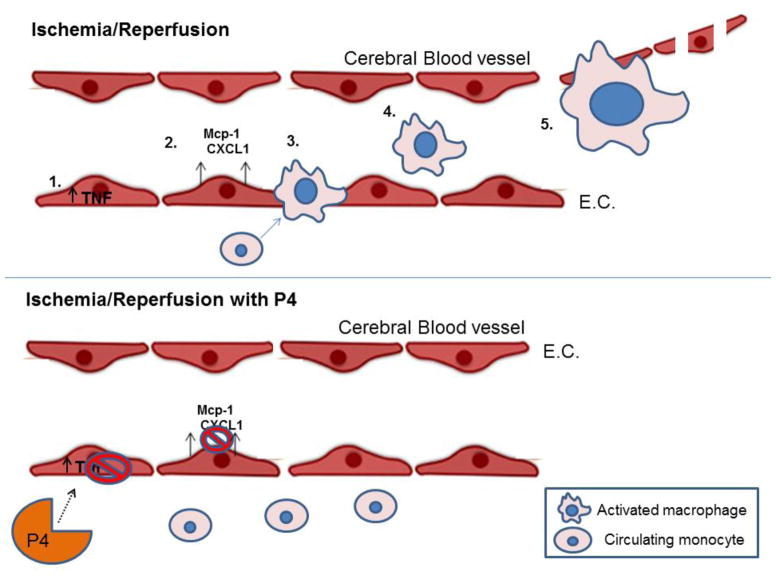
Figure 5.
Schematic of progesterone-mediated vascular protection after ischemia/reperfusion injury. After ischemia, TNF-α is upregulated within endothelial cells (1), leading to increased secretion of macrophage-recruiting chemokines MCP-1 and CXCL1 (2). Following chemokine upregulation, monocytes infiltrate the endothelial space, leading to the increased presence of macrophages (4) and macrophage-mediated endothelial cell damage (5). Progesterone’s effect on the cerebral vasculature after ischemia is to protect endothelial cells from further damage and loss by inhibiting TNF-alpha expression, chemokine secretion and macrophage infiltration.
Multiple reports clearly demonstrate that effective repair of ischemic tissue is dependent on a well-orchestrated cellular response and on timely induction and suppression of chemokines in a locally restricted manner. Our findings corroborate reports that early upregulation of chemotactic factors such as MCP-1 and CXCL1 in response to hypoxia/reoxygenation or ischemic/reperfusion injury contribute to ischemic injury through macrophage-mediated inflammatory signaling in the brain. The significance of our data is that progesterone-mediated suppression of secreted MCP-1 and CXCL1 will contribute to the protection of endothelial cells and ultimately blood vessels, which are necessary for CNS repair after stroke and subsequent functional recovery.
The results of this investigation provide a basis for further research into the potential therapeutic use of progesterone in patients suffering from cerebral and other forms of ischemia (e.g., renal ischemia) in which restoration of blood flow and vascular integrity would reduce injury and promote functional recovery.
Supplementary Material
A second antibody, anti-endothelial-barrier antigen (EBA) was used to confirm results from Figure 1 showing extensive injury-induced loss of endothelial cells 3 days following ischemia/reperfusion injury, which was attenuated by progesterone treatment. The antibodies were biotinylated anti-ABA (1:1,000 dilution; Abcam). Images were taken using confoncal microscopy at 20X.
Highlights.
Progesterone preserves vascularization after ischemic injury.
Progesterone protects endothelial cells from macrophage-induced cytotoxicity.
Progesterone decreases MCP-1- and CXCL1-dependent macrophage infiltration.
Vascular protective effect of progesterone is independent of angiogenesis.
Acknowledgments
SOURCES OF SUPPORT: This work was supported by NIH UO1 NS062676 to DGS and AHA SDG grant 11SDG5430002 to IS. Partial funding for some of the assays used in this project was provided by BHR Pharma and the Marcus Foundation.
Footnotes
The authors have no conflicts of interest concerning this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Hong A, Schulten H, Post MJ. Update on therapeutic neovascularization. Cardiovasc Res. 2005;65:639–648. doi: 10.1016/j.cardiores.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Prevalence of stroke--United States, 2006–2010. MMWR Morbidity and Mortality Weekly Report. 2012;61:379082. [PubMed] [Google Scholar]
- Centers for Disease Control. Vital signs: avoidable deaths from heart disease, stroke, and hypertensive disease - United States, 2001–2010. MMWR Morbidity and Mortality Weekly Report. 2013;62:721–727. [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. doi: 10.1242/dev.124.18.3633. [DOI] [PubMed] [Google Scholar]
- Farivar AS, Krishnadasan B, Naidu BV, Woolley SM, Verrier ED, Mulligan MS. Alpha chemokines regulate direct lung ischemia-reperfusion injury. J Heart Lung Transplant. 2004;23:585–591. doi: 10.1016/S1053-2498(03)00300-0. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. The role of the chemokines in myocardial ischemia and reperfusion. Curr Vasc Pharmacol. 2004;2:163–174. doi: 10.2174/1570161043476375. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Coomber B, Rathbone J. Is progesterone a candidate neuroprotective factor for treatment following ischemic stroke? Neuroscientist. 2009;15:324–332. doi: 10.1177/1073858409333069. [DOI] [PubMed] [Google Scholar]
- Grotta JC, Burgin WS, El-Mitwalli A, Long M, Campbell M, Morgenstern LB, Malkoff M, Alexandrov AV. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001;58:2009–2013. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- Hiu T, Nakagawa S, Hayashi K, et al. Tissue plasminogen activator enhances the hypoxia/reoxygenation-induced impairment of the blood-brain barrier in a primary culture of rat brain endothelial cells. Cell Mol Neurobiol. 2008;28:1139–1146. doi: 10.1007/s10571-008-9294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience. 2012;210:442–450. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm Res. 2009;58:619–624. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- Kaore SN, Langade DK, Yadav VK, Sharma P, Thawani VR, Sharma R. Novel actions of progesterone: what we know today and what will be the scenario in the future? J Pharm Pharmacol. 2012;64:1040–1062. doi: 10.1111/j.2042-7158.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Hirose M, Takahashi M, Kawaguchi M, Ise H, Kolattukudy PE, Yamada M, Ikeda U. MCP-1 induces cardioprotection against ischaemia/reperfusion injury: role of reactive oxygen species. Cardiovasc Res. 2008;78:554–562. doi: 10.1093/cvr/cvn035. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart-King CA. Endothelial cell adhesion and migration. Methods Enzymol. 2008;443:45–64. doi: 10.1016/S0076-6879(08)02003-X. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–159. [PubMed] [Google Scholar]
- Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- Shea-Donohue T, Thomas K, Cody MJ, Aiping Z, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124. doi: 10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroo I, Stokman G, Teske GJ, Raven A, Butter LM, Florquin S, Leemans JC. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int Immunol. 2010;22:433–442. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Sung FL, Zhu TY, Au-Yeung KK, Siow YL, OK Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB. Kidney Int. 2002;62:1160–1170. doi: 10.1111/j.1523-1755.2002.kid577.x. [DOI] [PubMed] [Google Scholar]
- Thom THN, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal KODC, Kitner S, Lloyd-Jones D, Goff DJ, Jr, Hong Y, Adams R, Friday GFK, Gorelik P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie PSJ, Wasserthiel-Smoller S, Wilson M, Wolff P. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Wali B, Ishrat T, Won S, Stein DG, Sayeed I. Progesterone in experimental permanent stroke: a dose-response and therapeutic time-window study. Brain. 2014;137:486–502. doi: 10.1093/brain/awt319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, Leeds P, Chuang DM. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke. 2012;43:2430–2436. doi: 10.1161/STROKEAHA.112.652545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S, Lee JH, Wali B, Stein DG, Sayeed I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway. J Cereb Blood Flow Metab. 2014;34:72–80. doi: 10.1038/jcbfm.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagnik DR, Hillyer P, Marshall D, Smythe CD, Krausz T, Haskard DO, Landis RC. Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis Rheum. 2000;43:1779–1789. doi: 10.1002/1529-0131(200008)43:8<1779::AID-ANR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yousuf S, Sayeed I, Atif F, Tang H, Wang J, Stein DG. Delayed progesterone treatment reduces brain infarction and improves functional outcomes after ischemic stroke: a time-window study in middle-aged rats. J Cereb Blood Flow Metab. 2014;34:297–306. doi: 10.1038/jcbfm.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A second antibody, anti-endothelial-barrier antigen (EBA) was used to confirm results from Figure 1 showing extensive injury-induced loss of endothelial cells 3 days following ischemia/reperfusion injury, which was attenuated by progesterone treatment. The antibodies were biotinylated anti-ABA (1:1,000 dilution; Abcam). Images were taken using confoncal microscopy at 20X.