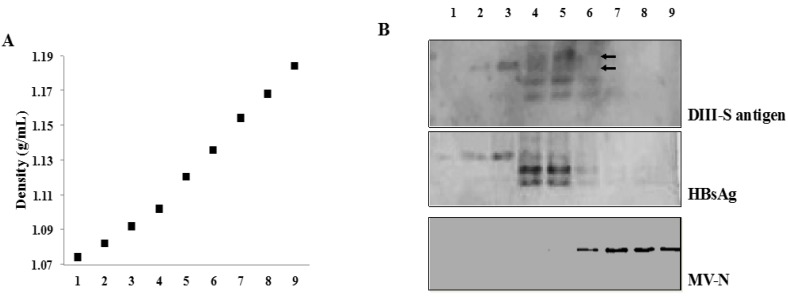
Figure 4.
(A) Materials released from Vero/hSLAM cells infected with MVvac2DIII-S,S)P or MVvac2(HBsAg)N collected 72 h after infection were clarified, and particulate material pelleted, loaded on a 20% to 60% sucrose gradient, and centrifuged to equilibrium. Five hundred microliter fractions were collected from the top (left) to the bottom (right) and weighed. The dotted line shows a representative density profile. (B) Aliquots of each fraction were separated by 12.5% SDS-polyacrylamide gel electrophoresis, immunoblotted, and probed with anti-HBsAg antibodies for fractions coming from cells infected with MVvac2DIII-S,S)P (upper panel) or MVvac2(HBsAg)N (medium panel). The lower panel indicates the migration of infectious MV virions.