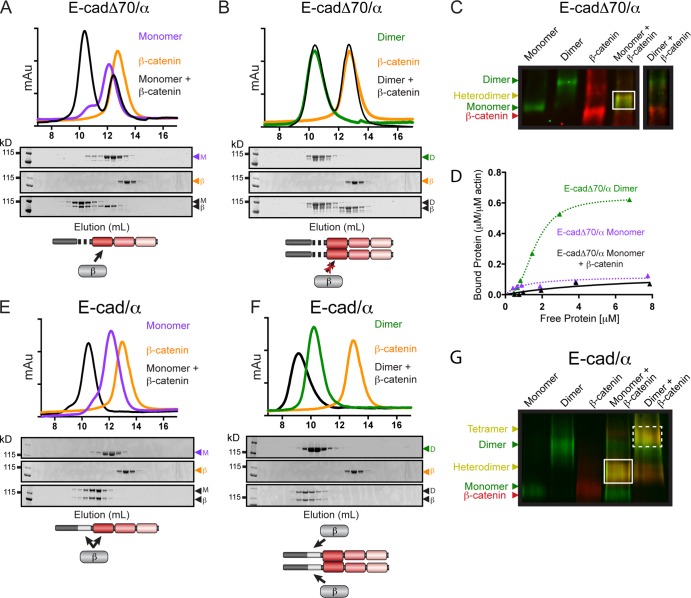
Figure 2.
Complex formation between E-cadherin/αE-catenin chimeras and β-catenin. Superdex 200 gel filtration chromatography of complex formation between β-catenin and E-cadΔ70/α monomer (A), E-cadΔ70/α homodimer (B), E-cad/α monomer (E), or E-cad/α homodimer (F). Proteins were incubated at 25°C either by themselves (colored lines) or together in a 1:1 molar ratio (black lines), and fractions from the individual S200 runs were analyzed by SDS-PAGE and CBB staining. A schematic representation of the complex formed between each chimera and β-catenin is shown. Native-PAGE of monomers and homodimers of E-cadΔ70/α (C) or E-cad/α (G) incubated with β-catenin and immunoblotted for αE-catenin (green) and β-catenin (red). Complex formation is indicated by a shift in band migration and co-fluorescence with both αE-catenin and β-catenin antibodies (heterodimer, solid box; tetramer, dashed box). Note that all proteins in C were run on the same Native-PAGE gel, but the brightness for the last lane was adjusted independently, as indicated. The gel images shown (C and G) are representative of four independent experiments. (D) High-speed co-sedimentation assay of E-cadΔ70/α-β-catenin heterodimer (black triangle) with F-actin. The data shown are from a single representative experiment out of three independent experiments. E-cadΔ70/α dimer (green triangle) and E-cadΔ70/α monomer (purple triangle) from F are shown for comparison.