Figure 5.
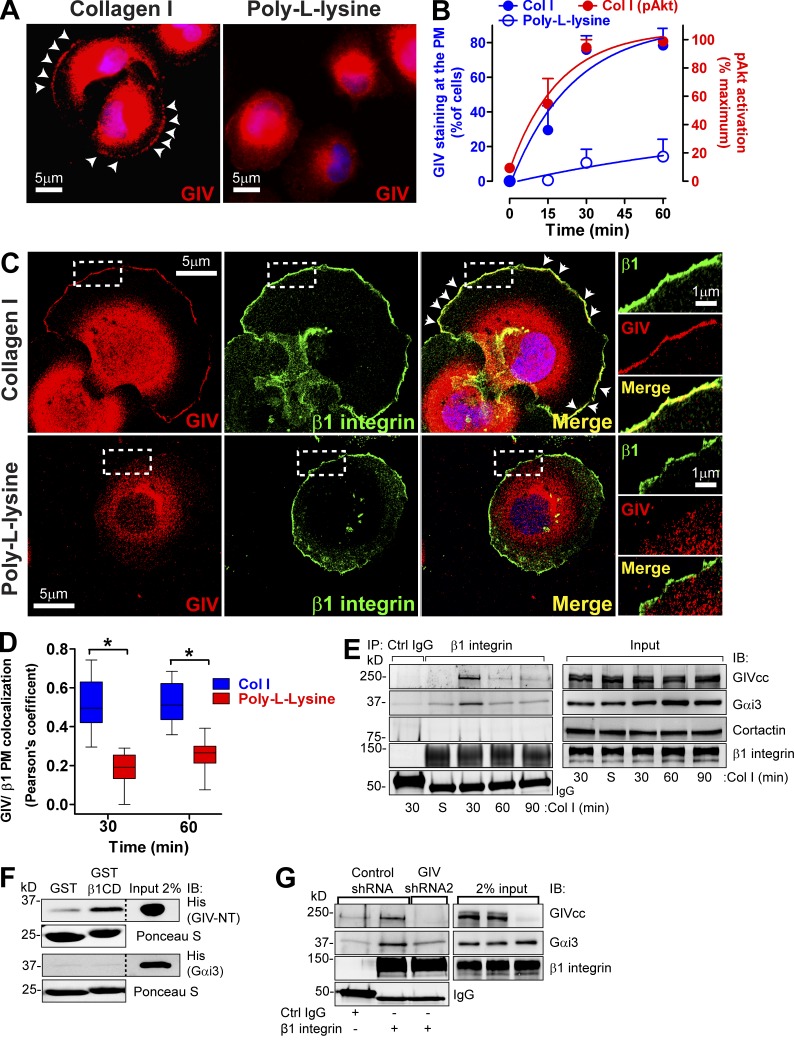
GIV is recruited to β1 integrins at the PM upon collagen I stimulation. (A and B) GIV is recruited to the cell periphery when cells attach to collagen I but not to poly-l-lysine. Control MDA-MB-231 cells were seeded on collagen I or the nonintegrin substrate poly-l-lysine following the protocol described in Fig. 2 B, except that the coated surfaces were glass coverslips and that cells were fixed and stained for GIV (red) or DNA (DAPI; blue). Representative pictures of the cells 60 min after seeding are shown in A. B shows the quantification of percentage of cells displaying GIV staining at the PM (blue; left axis) upon attachment to collagen I (filled circles) or poly-l-lysine (open circles). Results are depicted as mean ± SEM (error bars; n = 3–4). The quantification of Akt activation shown in Fig. 2 C is plotted here in red (right axis) for comparison. (C and D) GIV colocalizes with β1 integrin at the PM in cells attaching to collagen I but not to poly-l-lysine. Cells were treated as described in A but costained for GIV (red) and β1 integrin (green) and imaged by confocal microscopy. Representative pictures of cells 60 min after seeding are shown in C, and the quantification of three independent experiments are shown in D. White arrowheads indicate colocalization, and the boxed areas are shown enlarged on the right. Colocalization at the PM was quantified as described in Materials and methods and shown as box and whiskers plots (midline; median, box; 25–75%, whiskers; min-max range; n = 3; 8–10 cells/ experiment; *, P < 0.05). (E) GIV and Gαi3 coimmunoprecipitates with β1 integrins upon collagen I stimulation. Control MDA-MB-231 cells were seeded on collagen I for 30, 60, and 90 min as described in Fig. 2 B. Lysates of collagen I–attached cells or cells in suspension (S) were immunoprecipitated (IP) with β1-integrin antibodies (AIIB2) as described in Materials and methods. IPs (left) and lysates (right) were immunoblotted with the indicated antibodies. (F) GIV, but not Gαi3, directly binds to the cytoplasmic domain of β1 integrin (β1CD). Binding of purified His-GIV-NT (1–256) or His-Gαi3 to GST-β1CD was determined in pulldown assays as described in Materials and methods. (G) GIV depletion decreases Gαi3 coimmunoprecipitation with β1 integrins upon collagen I stimulation. MDA-MB-231 scr shRNA or GIV shRNA2 cells were seeded on collagen I for 30 min and immunoprecipitated as described in E.