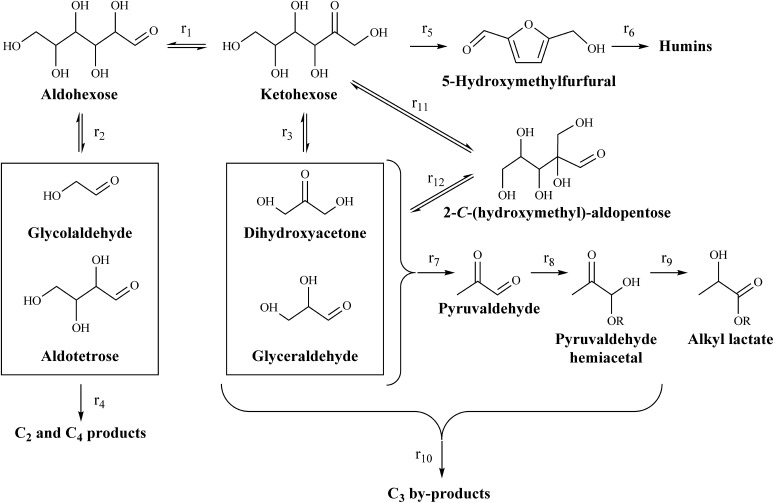
Fig. 1.
Schematic representation of reaction network in which ketohexoses can isomerize to aldohexoses via 1,2-HS (r1) and to 2-C-(hydroxymethyl)-aldopentoses via 1,2-CS (r11) reactions. Retro-aldol reactions of hexose species (r2, r3, and r12) lead to the formation of C2, C3, and C4 carbohydrate fragments. Lewis acids can then catalyze the formation of α-hydroxy carboxylic acids from these smaller fragments (e.g., r7, r8, and r9 in the formation of alkyl lactate from trioses). Side reactions, involving dehydration reactions of fructose to 5-HMF (r5), redox and fragmentation reactions of unstable intermediates, and various humin-forming condensation reactions, lead to loss of yield of desired products.